EX-99.1
Published on January 13, 2021

January 2021 NASDAQ: OPK

January 2021 | 2 This presentation contains “forward-looking statements,” as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA), which statements may be identified by words such as “expects,” “plans,” “projects,” “will,” “may,” “anticipates,” “believes,” “should,” “intends,” “estimates,” “potential,” and other words of similar meaning, including statements regarding expected financial performance and expectations regarding the market for and sales of our products, expectations about COVID-19 testing, the demand for testing, our capacity for testing and expected turnaround time, the impact of COVID-19 on all of our businesses, positively and negatively the success of our strategic ventures, whether the Scarlet Health mobile service and the integrated platform will function or perform as designed, the role and value of the service to patients and healthcare providers and whether there is a demand for the service or whether it will be available, our product development efforts and the expected benefits of our products, our estimated revenues and financial projections, whether we will maintain profitability or continue growth at BioReference, whether prescriptions for Rayaldee will continue to increase, the outcome of our clinical trials and validation studies and that such outcomes will support marketing approval or commercialization, the expected market penetration and size of the market for our products, our ability to successfully commercialize our product candidates such as hGH- CTP, Factor VII-CTP, and our rare disease product candidates, whether Rayaldee will raise serum total 25-hydroxyvitamin D (25D) more effectively than any over-the- counter (OTC) or prescription (Rx) products currently marketed without the risk of hypercalcemia, our ability to develop Rayaldee for new indications including stage 5 CKD, and the timeline for doing so, whether use of hGH-CTP in adults and pediatric patients will be approved, whether we will be required to make any changes to our development plans for hGH-CTP and increase our expenditures, the expected timing for commencing, enrolling, completing and announcing results for our clinical trials, the timing for release of trial data and seeking and obtaining FDA and foreign regulatory approvals as well as reimbursement coverage for our products. These forward-looking statements are only predictions and reflect our views as of the date they were made, and we undertake no obligation to update such statements. Such statements are subject to many risks and uncertainties that could cause our activities or actual results to differ materially from the activities and results anticipated in forward looking statements including the continued prevalence of COVID-19, developing and obtaining regulatory approvals of new, commercially viable and competitive products and treatments, the success of our collaboration and relationship with Pfizer and our other commercial partners, general market factors, competitive product development, product availability, federal and state regulations and legislation, delays associated with development of novel technologies, unexpected difficulties and delays in validating and testing product candidates, the regulatory process for new products and indications, the need for and availability of additional capital, the possibility of infringing a third party’s patents or other intellectual property rights, the uncertainty of obtaining patents covering our products and processes and in successfully enforcing them against third parties, and the potential for litigation or government investigations, including all of the risks identified under the heading Risk Factors in our Annual Report on Form 10-K and other filings with the Securities and Exchange and Commission. Forward-Looking Statements

January 2021 | 3 Pharmaceutical and Diagnostic platforms in growing healthcare markets Diagnostics Pharmaceuticals Third largest full service lab in US, 300- person commercial team drives industry- leading esoteric testing Best-in-breed genetic test offerings and most comprehensive menu in the industry, specializes in whole exome and genome sequencing and informatics Blood test for the detection of aggressive prostate cancer Approved for secondary HPT in Stage 3-4 CKD Cashflow supports 80-person commercial team Robust pipeline of rare disease drug candidates Two platform technologies enable novel long- acting therapeutics High unmet needs and long-dated IP Indication PC Ph 1 Ph 2 Ph 3 Rayaldee Stage 5 CKD Rayaldee COVID-19 Adult Growth Hormone Deficiency Pediatric Growth Hormone Deficiency Additional Pediatric Growth Hormone Disorders Hemophilia IGF-1 Deficiency Short Bowel Syndrome

January 2021 | 4 Somatrogon© Summary • Somatrogon© (hGH-CTP), a once-weekly growth hormone replacement therapy, was proven non-inferior to daily Genotropin® (somatropin) with respect to height velocity after 12 months • Height velocity at 12 months of treatment was higher in the somatrogon© group (10.12 cm/year) than in the somatropin group (9.78 cm/year) PEDIATRIC INDICATION-ACHIEVED PRIMARY ENDPOINT • Change in height standard deviation scores at six and 12 months were higher with somatrogon© in comparison to somatropin • At six months, change in height velocity was higher with somatrogon© in comparison to somatropin • Somatrogon© was generally well tolerated in the study and comparable to that of somatropin dosed once-daily with respect to the types, numbers and severity of the adverse events observed between the treatment arms. PEDIATRIC INDICATION-SECONDARY ENDPOINTS ACHIEVED GLOBAL PARTNERSHIP WITH PFIZER • Pfizer to commercialize somatrogon© • Highly committed to maintaining global hGH franchise • Height velocity at 12 months of treatment was higher in the somatrogon© group (9.65 cm/year) than in the somatropin group (7.87 cm/year) • Study met all primary and secondary endpoints PEDIATRIC INDICATION-SUCCESSFUL JAPANESE REGISTRATION STUDY • Treatment with somatrogon© once-weekly improved the mean overall Life Interference total score after 12 weeks of treatment compared to treatment with somatropin administered once-daily. • Key secondary endpoints showed an overall benefit in treatment experience with the somatrogon© once-weekly dosing regimen compared to the somatropin once-daily dosing regimen PEDIATRIC INDICATION-SUCCESSFUL PHASE 3 CROSSOVER STUDY U.S. BLA SUBMISSION ACCEPTED • Prescription Drug User Fee Act (PDUFA) date in October 2021
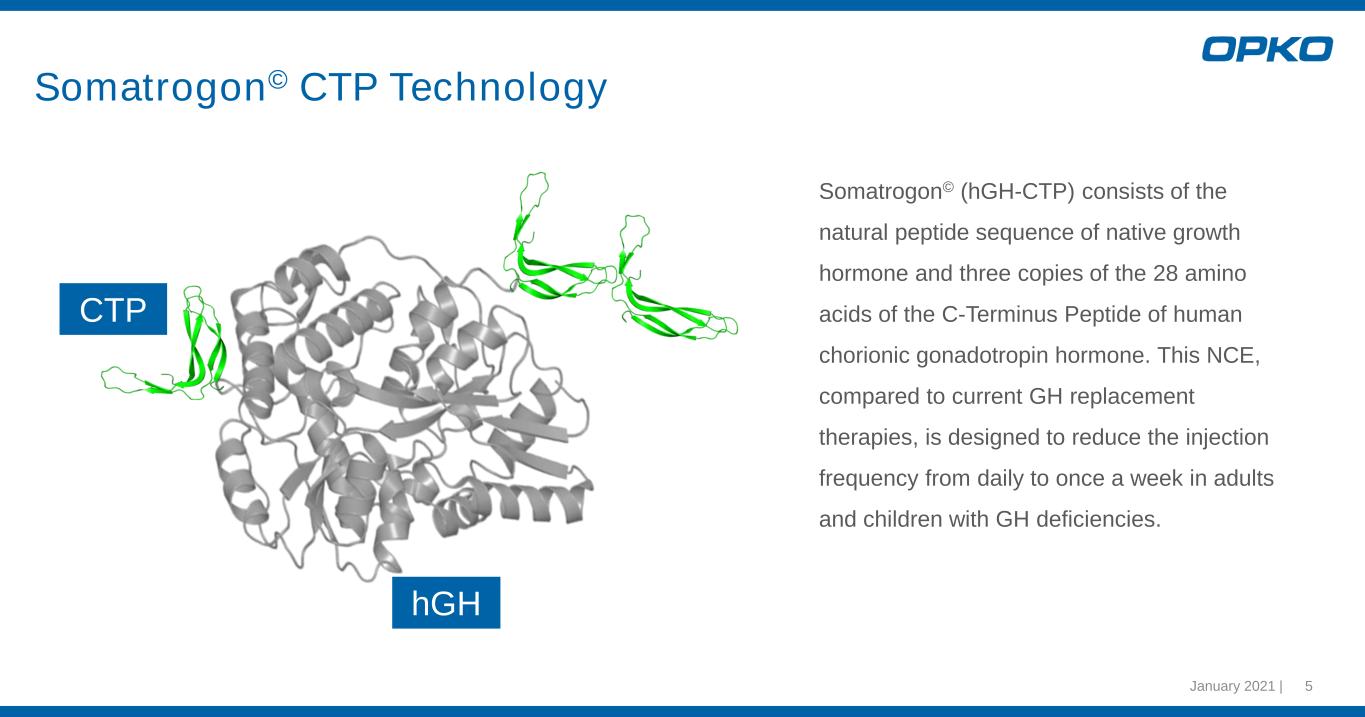
Somatrogon© (hGH-CTP) consists of the natural peptide sequence of native growth hormone and three copies of the 28 amino acids of the C-Terminus Peptide of human chorionic gonadotropin hormone. This NCE, compared to current GH replacement therapies, is designed to reduce the injection frequency from daily to once a week in adults and children with GH deficiencies. January 2021 | 5 Somatrogon© CTP Technology CTP hGH
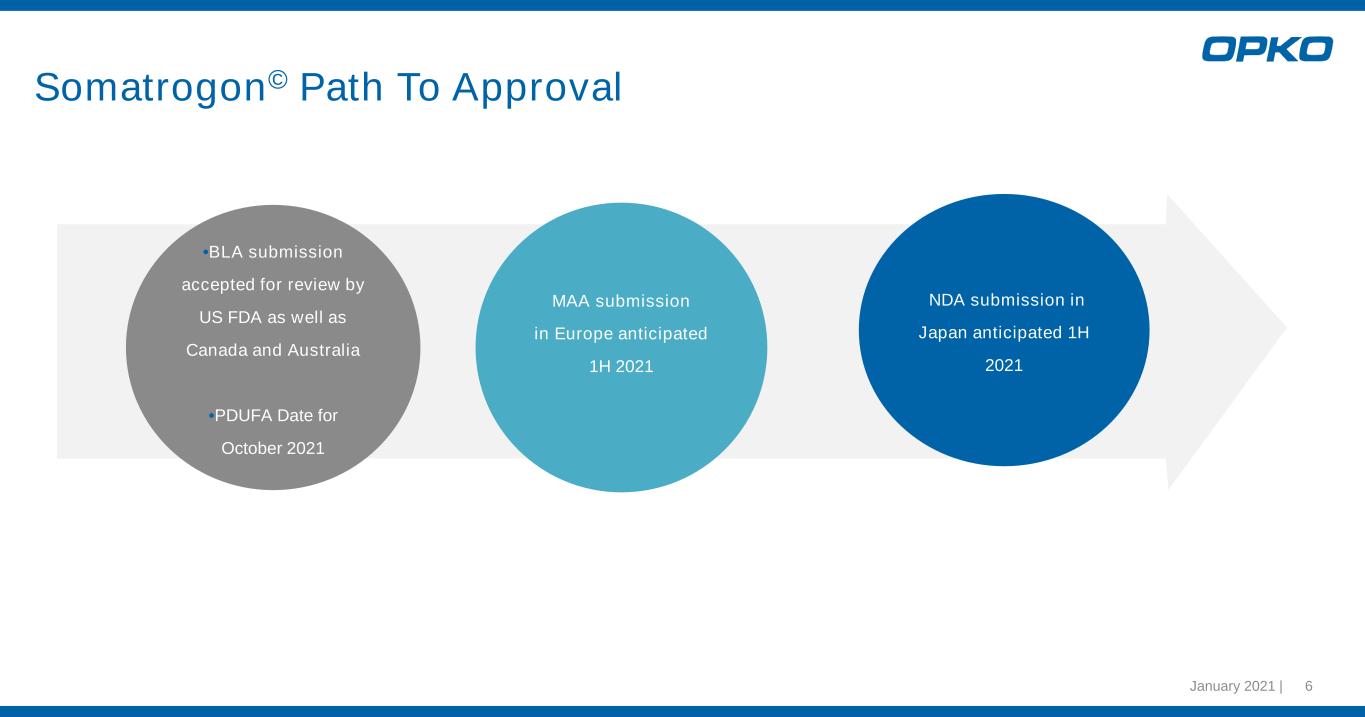
January 2021 | 6 Somatrogon© Path To Approval •BLA submission accepted for review by US FDA as well as Canada and Australia •PDUFA Date for October 2021 •NDA submission in Japan anticipated 1H 2021 MAA submission in Europe anticipated 1H 2021

January 2021 | 7 Diversified Assets Marketed Pharmaceuticals Pipeline Diagnostics
• Once daily oral formulation of the prohormone 25D3* addresses significant unmet need • Only product approved by FDA to treat secondary hyperparathyroidism (SHPT) in patients with stage 3-4 CKD and vitamin D insufficiency • Reduces plasma iPTH and increases serum 25D, with safety profile similar to placebo • Minimal adverse effects on serum calcium or phosphorus, key drivers of vascular calcification • Total prescriptions of Rayaldee increased 13%** in 3Q 2020 compared to 3Q 2019 and were consistent with the second quarter 2Q 2020 despite COVID- 19 restrictions January 2021 | 8 First and only extended-release prohormone of active form of vitamin D3 * 25-Hydroxyvitamin D3 or Calcifediol Chronic Kidney Disease (CKD) The Silent Killer • 9th leading cause of death, ahead of breast and prostate cancer • Prevalence expected to increase due to obesity, diabetes and hypertension • Elevated blood levels of intact parathyroid hormone (iPTH) arise from vitamin D insufficiency • High PTH levels promote vascular calcification, a major cause of CKD morbidity and mortality • Updated KDIGO practice guideline recommends against routine use of vitamin D receptor activators and highlights unproven effectiveness of vitamin D supplements (ie., cholecalciferol and ergocalciferol) Healthcare providers have no good options to treat SHPT in stage 3-4 CKD except for Rayaldee ** As reported by IQVIA
• Commenced September 2018 • Approximately 44 patients treated for 26 weeks • Interim data released in March 2020 • Topline data expected in 1Q 2021 • Costs shared with Vifor Fresenius and Japan Tobacco January 2021 | 9 Rayaldee for Patients with Stage 5 CKD and Vitamin D Insuffiency Phase 2 open-label trial to evaluate the safety, efficacy, pharmacokinetics and pharmacodynamics of higher strength Rayaldee in subjects with Vitamin D insufficiency and CKD requiring regular hemodialysis.
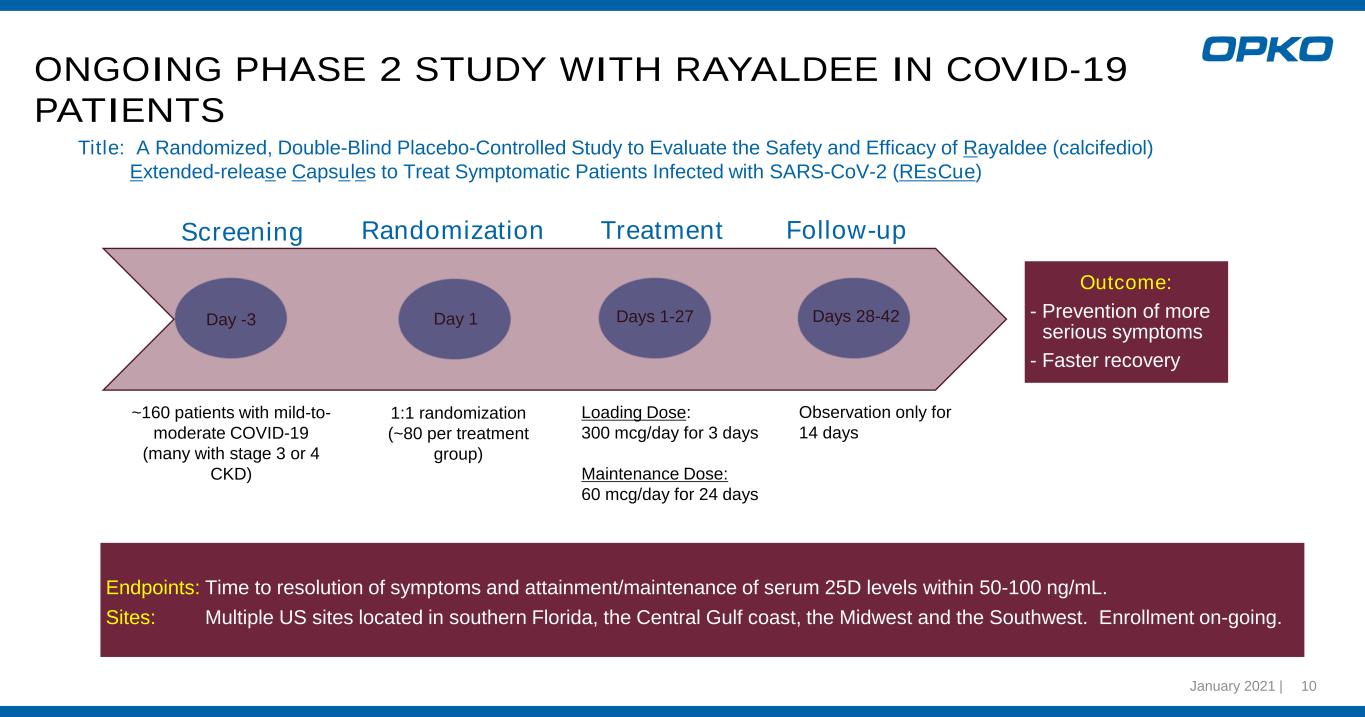
January 2021 | 10 ONGOING PHASE 2 STUDY WITH RAYALDEE IN COVID-19 PATIENTS Outcome: - Prevention of more serious symptoms - Faster recovery Day -3 Day -3 Days 1-27 Days 28-42 Screening Randomization Treatment Follow-up Title: A Randomized, Double-Blind Placebo-Controlled Study to Evaluate the Safety and Efficacy of Rayaldee (calcifediol) Extended-release Capsules to Treat Symptomatic Patients Infected with SARS-CoV-2 (REsCue) ~160 patients with mild-to- moderate COVID-19 (many with stage 3 or 4 CKD) 1:1 randomization (~80 per treatment group) Loading Dose: 300 mcg/day for 3 days Maintenance Dose: 60 mcg/day for 24 days Day 1 Endpoints: Time to resolution of symptoms and attainment/maintenance of serum 25D levels within 50-100 ng/mL. Sites: Multiple US sites located in southern Florida, the Central Gulf coast, the Midwest and the Southwest. Enrollment on-going. Observation only for 14 days

January 2021 | 11 Diversified Assets Broad Development Pipeline Marketed Pharmaceuticals DiagnosticsPipeline
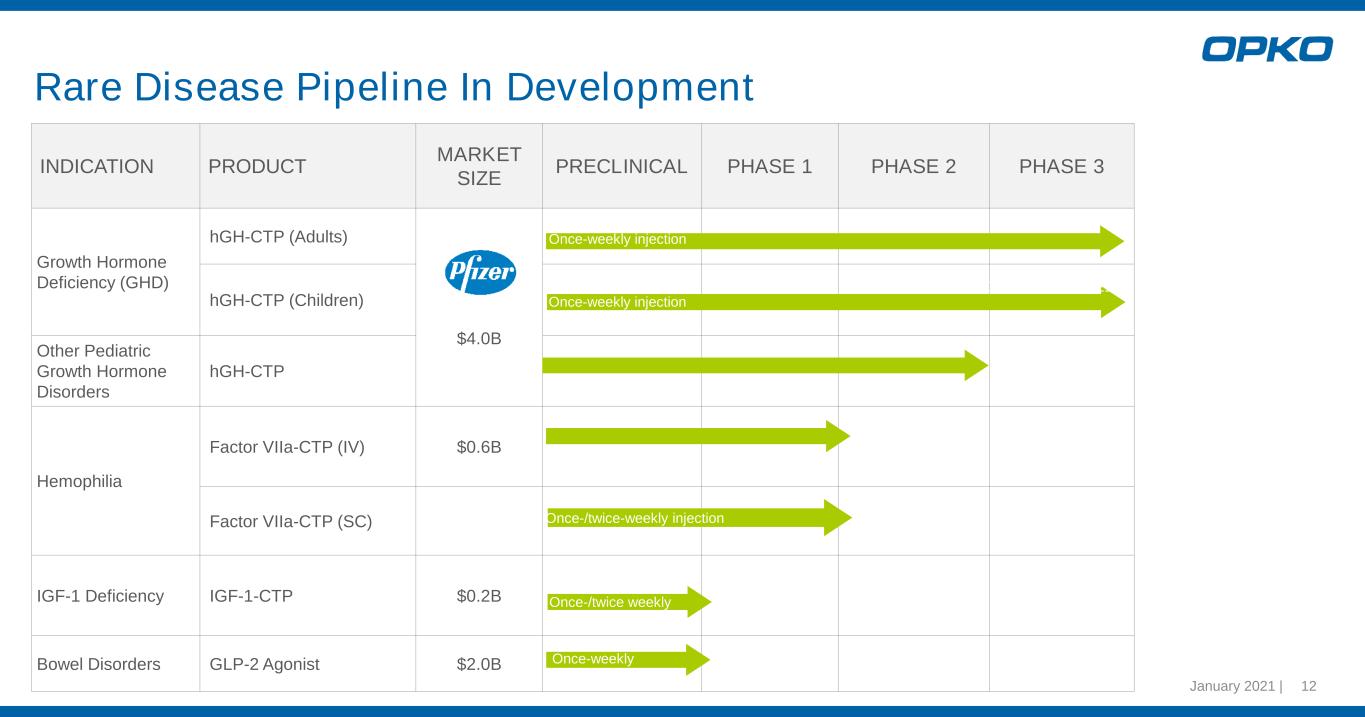
INDICATION PRODUCT MARKET SIZE PRECLINICAL PHASE 1 PHASE 2 PHASE 3 Growth Hormone Deficiency (GHD) hGH-CTP (Adults) $4.0B hGH-CTP (Children) Other Pediatric Growth Hormone Disorders hGH-CTP Hemophilia Factor VIIa-CTP (IV) $0.6B Factor VIIa-CTP (SC) IGF-1 Deficiency IGF-1-CTP $0.2B Bowel Disorders GLP-2 Agonist $2.0B Once-weekly injection Once-weekly injection Once-/twice-weekly injection Once-weekly PDUFA October 2021 Once-/twice weekly Rare Disease Pipeline In Development January 2021 | 12

January 2021 | 13 Diversified Assets One of the Nation’s Largest Reference Laboratories Marketed Pharmaceuticals DiagnosticsPipeline
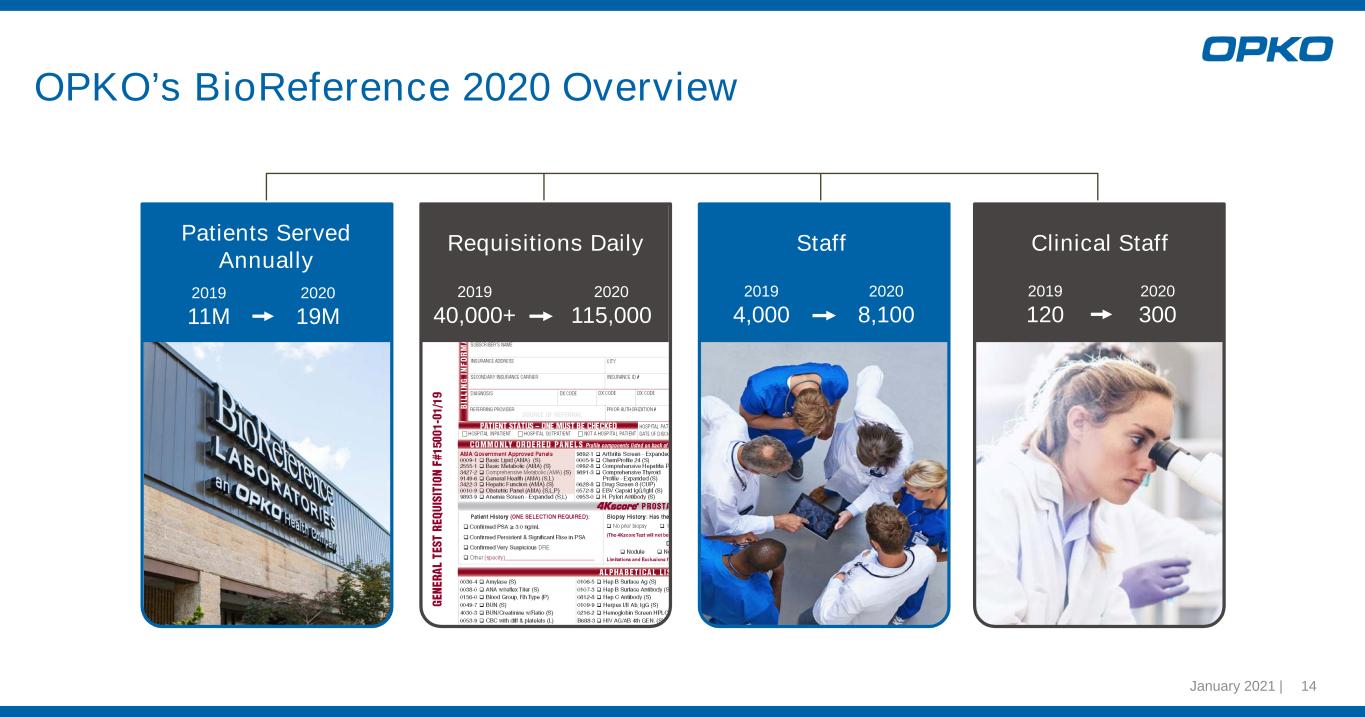
January 2021 | 14 OPKO’s BioReference 2020 Overview Patients Served Annually Requisitions Daily 2019 40,000+ 2020 115,000 2019 4,000 2020 8,100 Staff Clinical Staff 2019 11M 2020 19M 2019 120 2020 300

January 2021 | 15 Areas of Focus with Customized Solutions COVID-19 Digital Health Core Business Strategic Venture Services

16 COVID-19 Response Physicians & Hospitals January 2021 | Education GovernmentSports Employers RetailTravel & Leisure Large scale screening and point-of-care testing

January 2021 | 17 NFL Enhancement

18*numbers as of January. 2021 COVID-19 Diagnostic Offerings 10,000,000+ Molecular Tests Performed* 100,000+ Molecular Tests Per Day Capacity* 60,000+ Point-of-Care Tests Performed* January 2021 |

19 The Future of COVID-19 Testing for 2021 • Many factors continue to drive demand for COVID-19 testing at BioReference Laboratories. o Retail access remains imperative for the general public. o Physicians will continue to test their patients. o Employers are working through their strategies for employees to return-to the-office. o Transportation and hospitality will continue to need testing. o Schools and universities will continue to need testing. o Testing strategies as related to improving the economy will continue to be needed. January 2021 | BioReference will continue to lead the industry with custom solutions, point-of-care testing and large-scale screening programs

January 2021 | 20 Core Business Areas Clinical Women’s Health Oncology Urology Genetics

January 2021 | 21 Digital Health
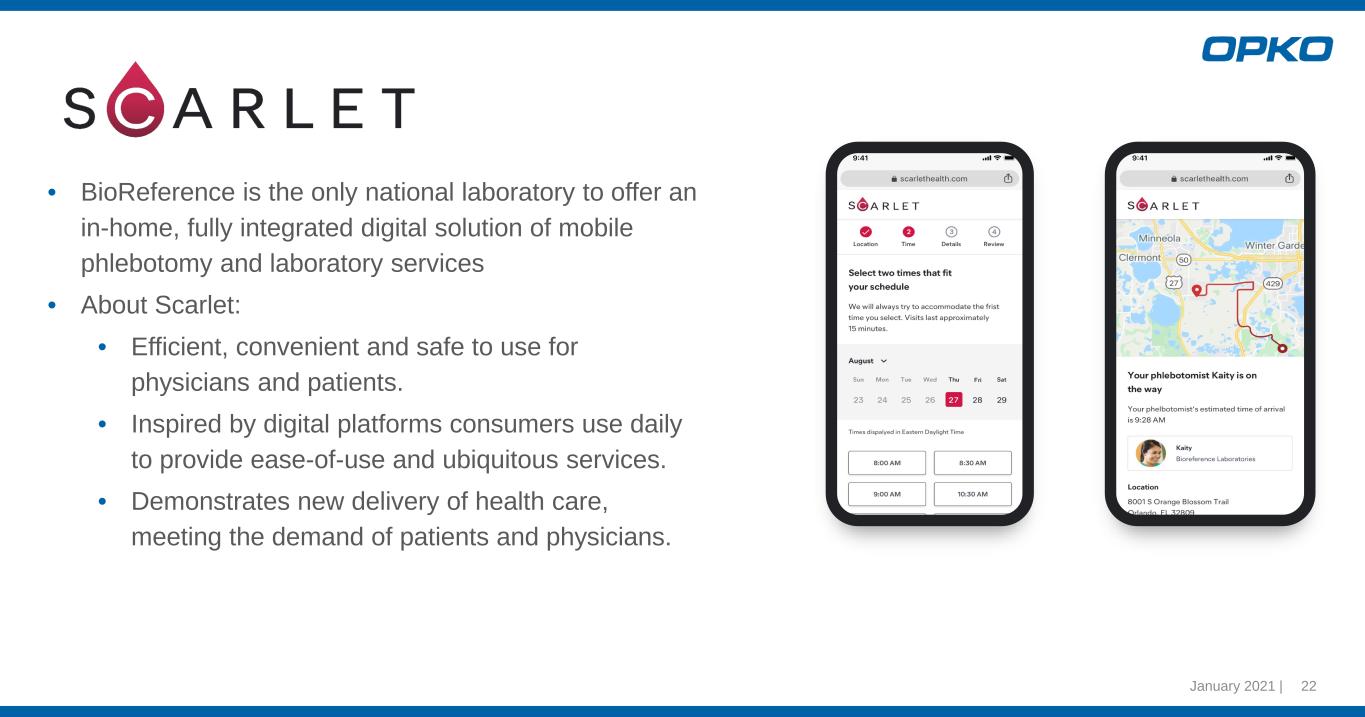
• BioReference is the only national laboratory to offer an in-home, fully integrated digital solution of mobile phlebotomy and laboratory services • About Scarlet: • Efficient, convenient and safe to use for physicians and patients. • Inspired by digital platforms consumers use daily to provide ease-of-use and ubiquitous services. • Demonstrates new delivery of health care, meeting the demand of patients and physicians. January 2021 | 22
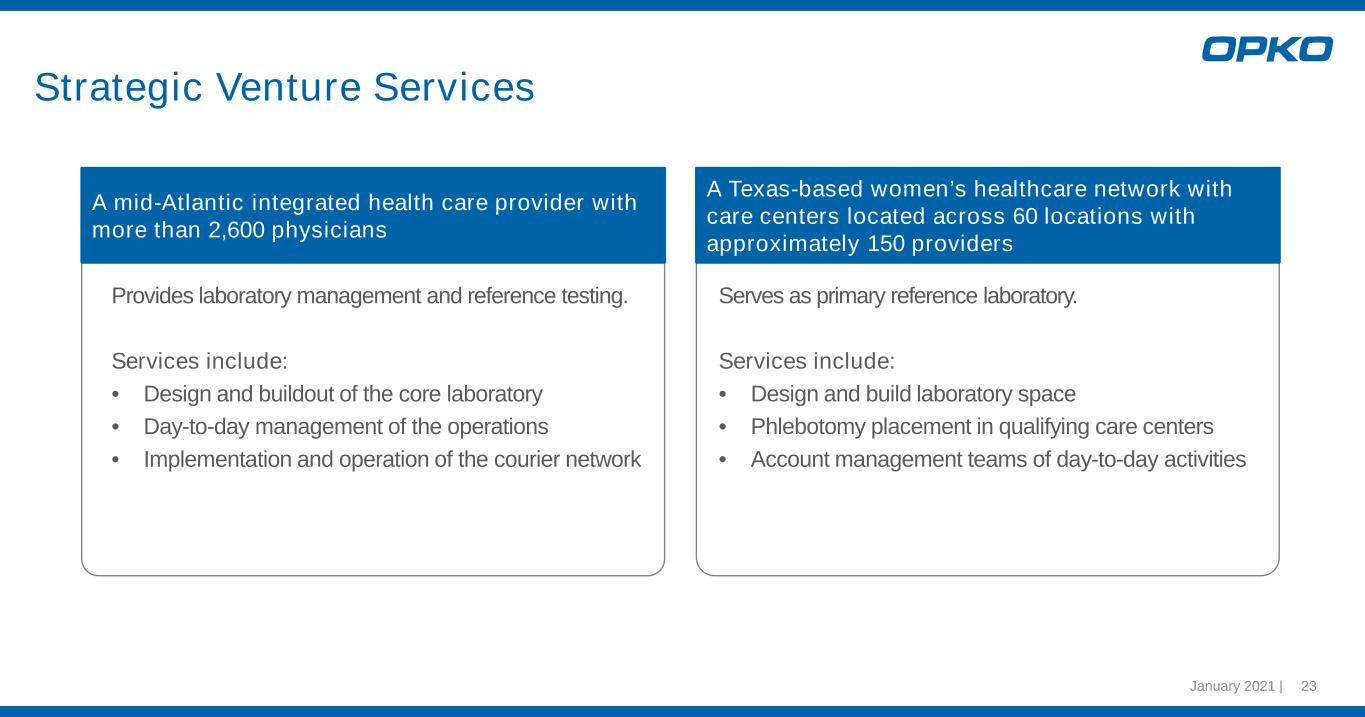
January 2021 | 23 Strategic Venture Services A mid-Atlantic integrated health care provider with more than 2,600 physicians Provides laboratory management and reference testing. Services include: • Design and buildout of the core laboratory • Day-to-day management of the operations • Implementation and operation of the courier network A Texas-based women’s healthcare network with care centers located across 60 locations with approximately 150 providers Serves as primary reference laboratory. Services include: • Design and build laboratory space • Phlebotomy placement in qualifying care centers • Account management teams of day-to-day activities
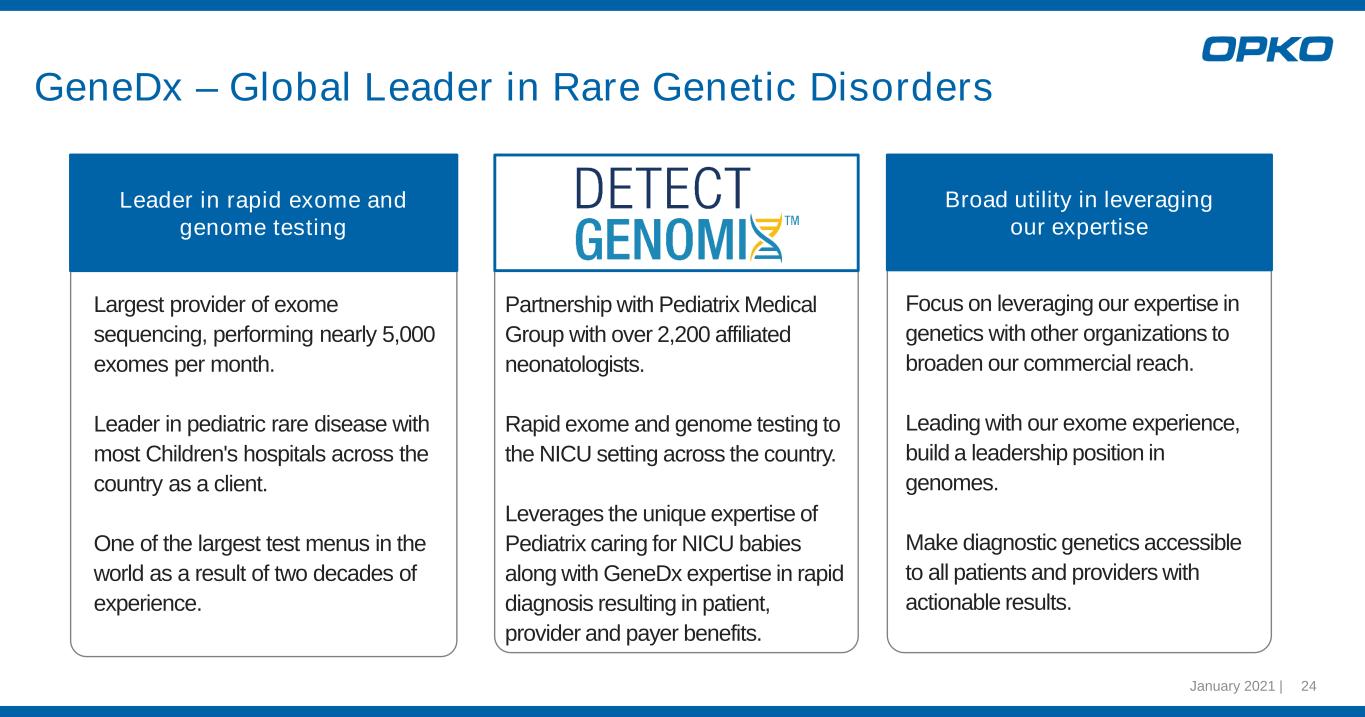
January 2021 | 24 GeneDx – Global Leader in Rare Genetic Disorders Leader in rapid exome and genome testing Largest provider of exome sequencing, performing nearly 5,000 exomes per month. Leader in pediatric rare disease with most Children's hospitals across the country as a client. One of the largest test menus in the world as a result of two decades of experience. Partnership with Pediatrix Medical Group with over 2,200 affiliated neonatologists. Rapid exome and genome testing to the NICU setting across the country. Leverages the unique expertise of Pediatrix caring for NICU babies along with GeneDx expertise in rapid diagnosis resulting in patient, provider and payer benefits. Broad utility in leveraging our expertise Focus on leveraging our expertise in genetics with other organizations to broaden our commercial reach. Leading with our exome experience, build a leadership position in genomes. Make diagnostic genetics accessible to all patients and providers with actionable results.
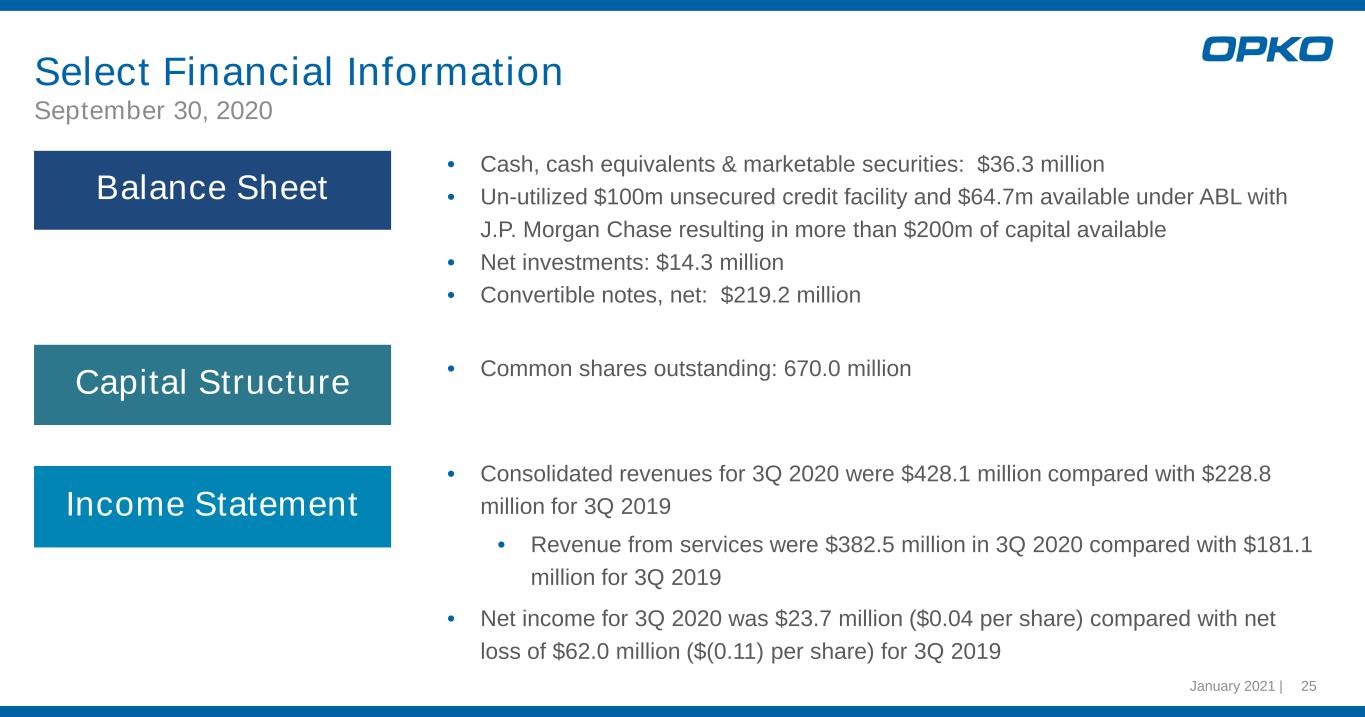
January 2021 | 25 Select Financial Information September 30, 2020 • Cash, cash equivalents & marketable securities: $36.3 million • Un-utilized $100m unsecured credit facility and $64.7m available under ABL with J.P. Morgan Chase resulting in more than $200m of capital available • Net investments: $14.3 million • Convertible notes, net: $219.2 million • Common shares outstanding: 670.0 million • Consolidated revenues for 3Q 2020 were $428.1 million compared with $228.8 million for 3Q 2019 • Revenue from services were $382.5 million in 3Q 2020 compared with $181.1 million for 3Q 2019 • Net income for 3Q 2020 was $23.7 million ($0.04 per share) compared with net loss of $62.0 million ($(0.11) per share) for 3Q 2019 Balance Sheet Capital Structure Income Statement