EX-99.2
Published on September 24, 2009
Exhibit 99.2

| Jamie Freedman M.D., Ph.D.Executive Vice President, Research & Development OPKO Health, Inc.Miami, Florida The UBS Life Sciences MeetingNew York, Sept. 22, 2009 |

| Cautionary Statement 2 This presentation contains "forward-looking statements," as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA), which statements may be identified by words such as "expects," "plans," "projects," "will," "may," "anticipates," "believes," "should," "intends," "estimates," and other words of similar meaning, including statements regarding our ability to build a diverse portfolio of important medical products with significant commercial value, our product development efforts and expected timing thereof, our ability to expand applications and increase sales of ophthalmic instruments worldwide, expectations regarding the commercialization of bevasiranib and our other products, the products' potential benefits, statements regarding the timing of clinical trials for our product candidates, estimates regarding market potential and timing of regulatory approval for our product candidates, our ability to invest in Research and Development, our ability to explore innovative drug delivery systems for RNA interference, demonstrate clinical proof-of-concept with early clinical products, make strategic acquisitions of late stage clinical products and opportunistic acquisitions of mature pharmaceutical businesses, and develop technologies for early detection of diseases such as Alzheimer's and cancer, as well as other non-historical statements. These forward-looking statements are only predictions and reflect our views as of the date they were made, and we undertake no obligation to update such statements. Such statements are subject to many risks and uncertainties that could cause our actual activities or results to differ materially from the activities and results anticipated in forward-looking statements, including risks inherent in funding, developing and obtaining regulatory approvals of new, commercially-viable and competitive products and treatments, general market factors, competitive product development, product availability, federal and state regulations and legislation, the regulatory process for new products and indications, manufacturing issues that may arise, the possibility of infringing a third party's patents or other intellectual property rights, the uncertainty of obtaining patents covering our products and processes and in successfully enforcing them against third parties, and the possibility of litigation, among other factors. |

| OPKO Health, Inc. A specialty healthcare company building a diverse portfolio of important medical products with significant commercial value. 3 |
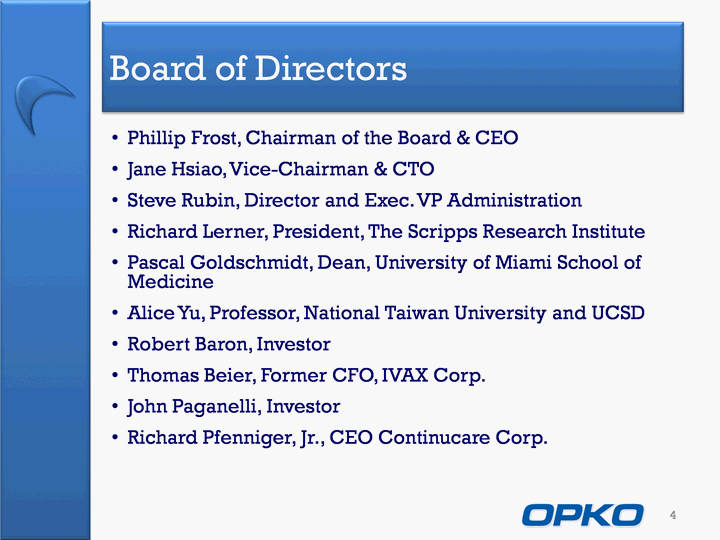
| Board of Directors Phillip Frost, Chairman of the Board & CEOJane Hsiao, Vice-Chairman & CTOSteve Rubin, Director and Exec. VP Administration Richard Lerner, President, The Scripps Research InstitutePascal Goldschmidt, Dean, University of Miami School of MedicineAlice Yu, Professor, National Taiwan University and UCSDRobert Baron, InvestorThomas Beier, Former CFO, IVAX Corp.John Paganelli, InvestorRichard Pfenniger, Jr., CEO Continucare Corp. 4 |

| Management Team Dr. Phillip Frost, CEO & Chairman of the BoardDr. Jane Hsiao, Vice-Chairman & Chief Technical OfficerMr. Steve Rubin, Exec. VP, AdministrationDr. Rao Uppaluri, Sr. VP & Chief Financial OfficerDr. Jamie Freedman, Exec. VP R&D 5 |
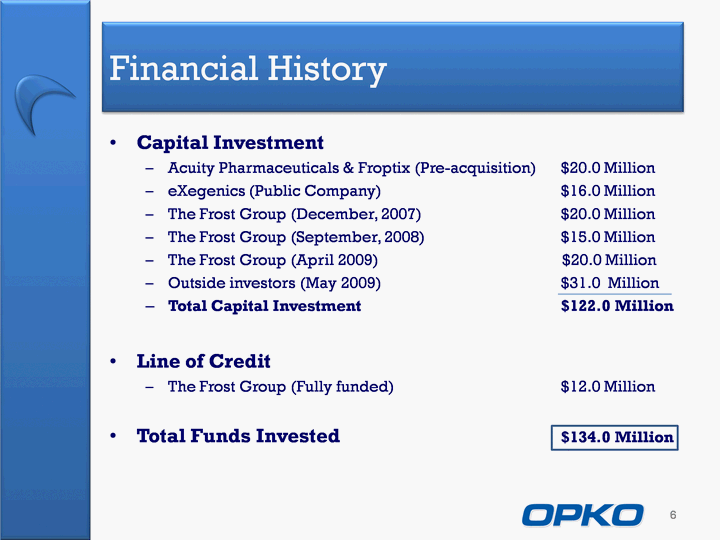
| Financial History Capital InvestmentAcuity Pharmaceuticals & Froptix (Pre-acquisition) $20.0 MillioneXegenics (Public Company) $16.0 MillionThe Frost Group (December, 2007) $20.0 MillionThe Frost Group (September, 2008) $15.0 MillionThe Frost Group (April 2009) $20.0 MillionOutside investors (May 2009) $31.0 MillionTotal Capital Investment $122.0 MillionLine of CreditThe Frost Group (Fully funded) $12.0 MillionTotal Funds Invested $134.0 Million 6 |
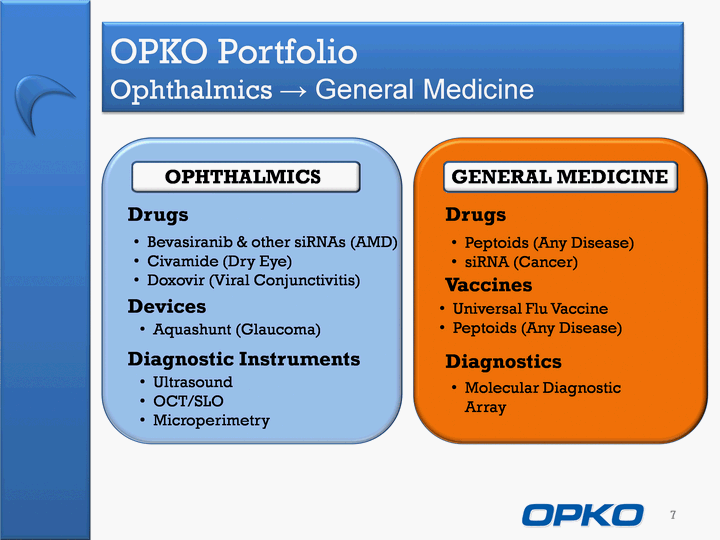
| OPKO Portfolio Ophthalmics ^ General Medicine 7 OPHTHALMICS GENERAL MEDICINE Drugs Drugs Vaccines Diagnostics Devices Diagnostic Instruments Bevasiranib & other siRNAs (AMD) Civamide (Dry Eye)Doxovir (Viral Conjunctivitis) Aquashunt (Glaucoma) UltrasoundOCT/SLOMicroperimetry Molecular Diagnostic Array Peptoids (Any Disease)siRNA (Cancer) Universal Flu VaccinePeptoids (Any Disease) |
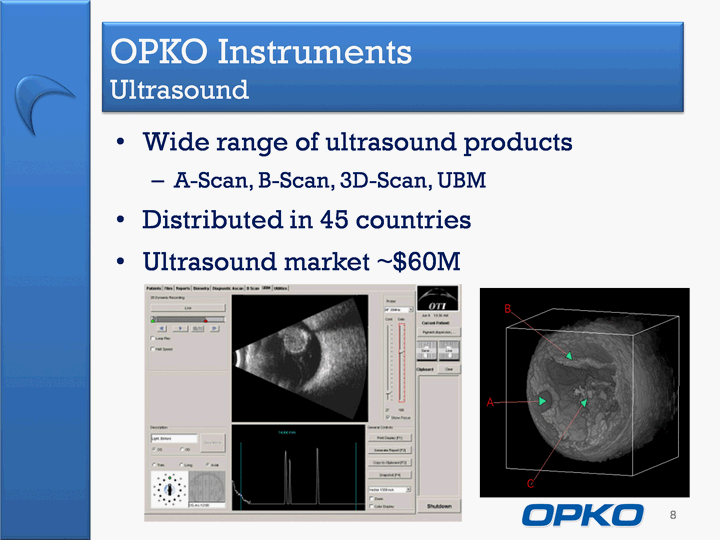
| Wide range of ultrasound productsA-Scan, B-Scan, 3D-Scan, UBMDistributed in 45 countriesUltrasound market ~$60M OPKO Instruments Ultrasound 8 |
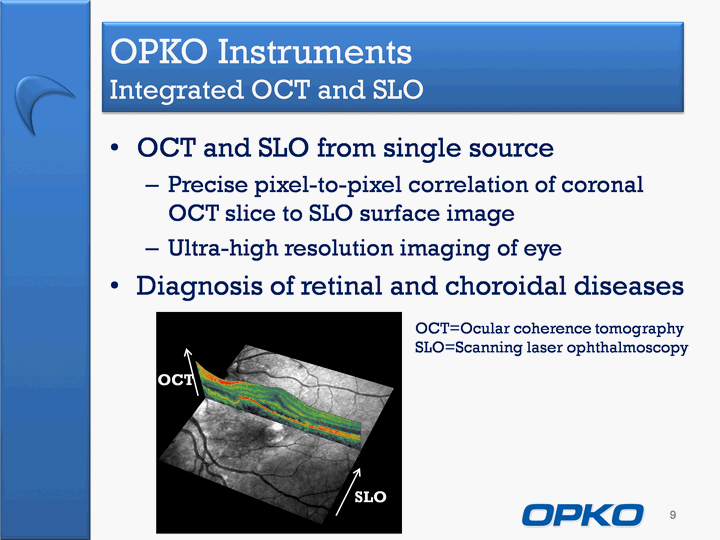
| OCT and SLO from single sourcePrecise pixel-to-pixel correlation of coronal OCT slice to SLO surface imageUltra-high resolution imaging of eyeDiagnosis of retinal and choroidal diseases 9 OPKO Instruments Integrated OCT and SLO OCT SLO OCT=Ocular coherence tomography SLO=Scanning laser ophthalmoscopy |
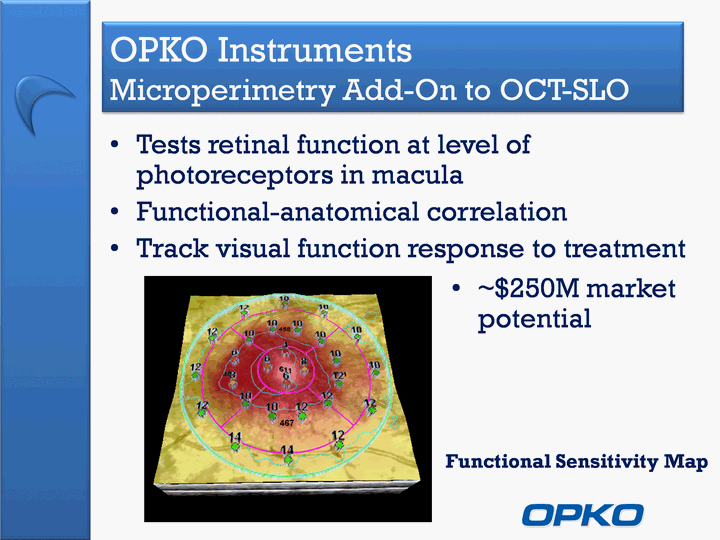
| OPKO Instruments Microperimetry Add-On to OCT-SLO Tests retinal function at level of photoreceptors in maculaFunctional-anatomical correlationTrack visual function response to treatment Functional Sensitivity Map ~$250M market potential |
| Molecular Diagnostics Overview Global molecular diagnostic market projected to reach ~$4B by 2010Largest growth segments:Early detectionCompanion diagnostics 11 |
| Molecular Diagnostics Small Molecule Microarray Molecular microarray technology acquired June 17, 2009"Peptoids" as first example of small molecule array to detect disease- associated antibodiesBroad application for diagnosing diseases by a simple blood testNeurological disorders (Alzheimers Disease)Cancers (Lung Cancer)Other Diseases 12 |
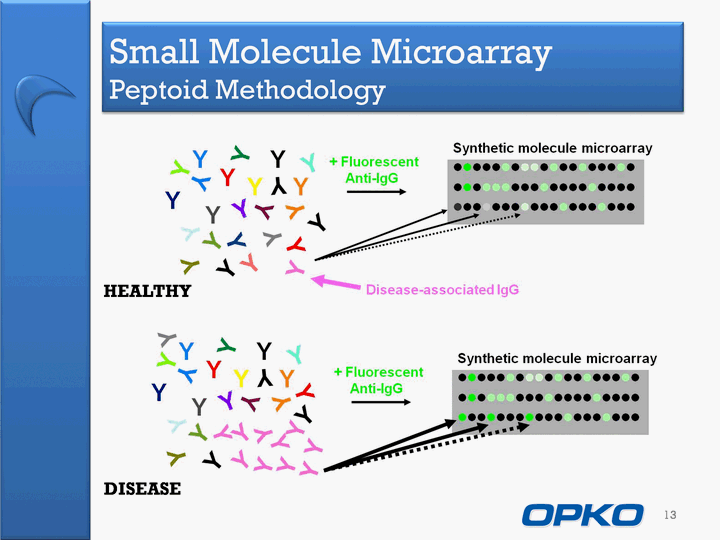
| Small Molecule Microarray Peptoid Methodology 13 HEALTHY DISEASE |
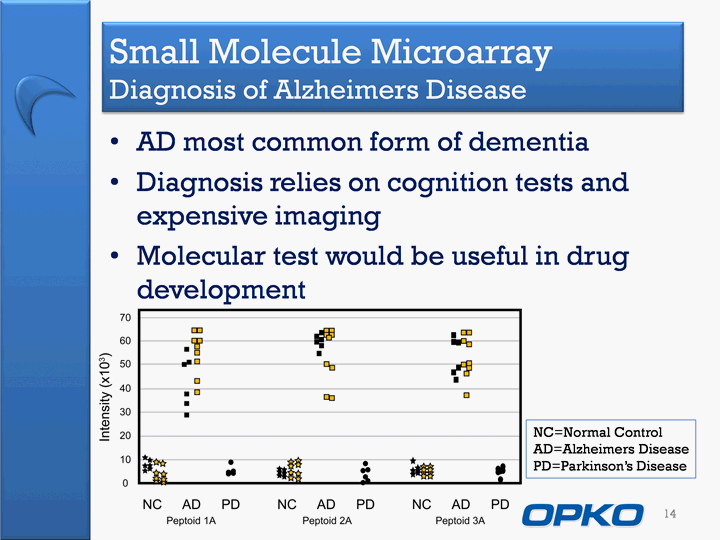
| Small Molecule Microarray Diagnosis of Alzheimers Disease AD most common form of dementiaDiagnosis relies on cognition tests and expensive imagingMolecular test would be useful in drug development 14 NC=Normal ControlAD=Alzheimers DiseasePD=Parkinson's Disease |
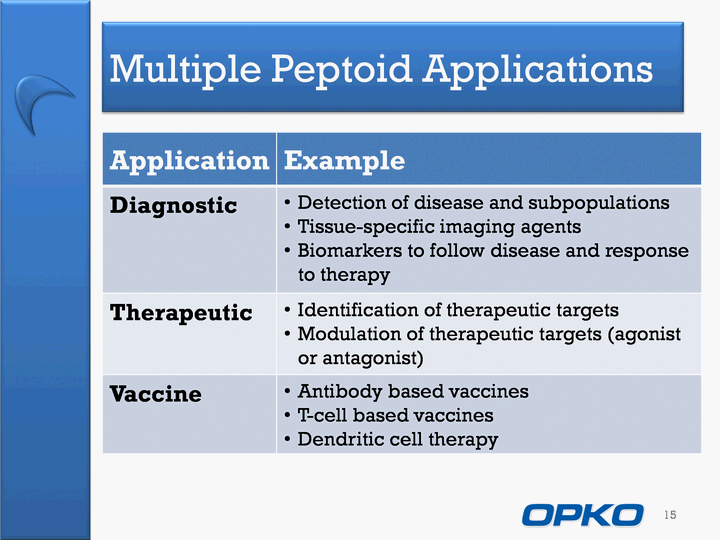
| Multiple Peptoid Applications 15 Application Example Diagnostic Detection of disease and subpopulationsTissue-specific imaging agentsBiomarkers to follow disease and response to therapy Therapeutic Identification of therapeutic targetsModulation of therapeutic targets (agonist or antagonist) Vaccine Antibody based vaccinesT-cell based vaccinesDendritic cell therapy |
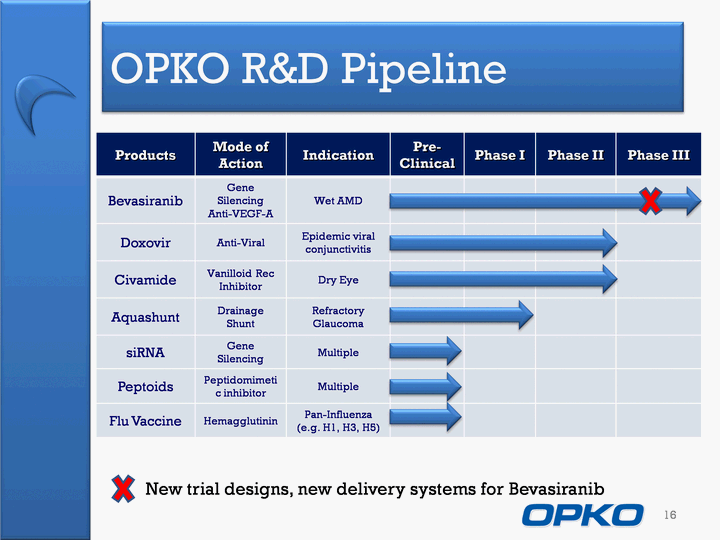
| OPKO R&D Pipeline 16 Products Mode of Action Indication Pre-Clinical Phase I Phase II Phase III Bevasiranib Gene SilencingAnti-VEGF-A Wet AMD Doxovir Anti-Viral Epidemic viral conjunctivitis Civamide Vanilloid Rec Inhibitor Dry Eye Aquashunt Drainage Shunt Refractory Glaucoma siRNA Gene Silencing Multiple Peptoids Peptidomimetic inhibitor Multiple Flu Vaccine Hemagglutinin Pan-Influenza (e.g. H1, H3, H5) New trial designs, new delivery systems for Bevasiranib |
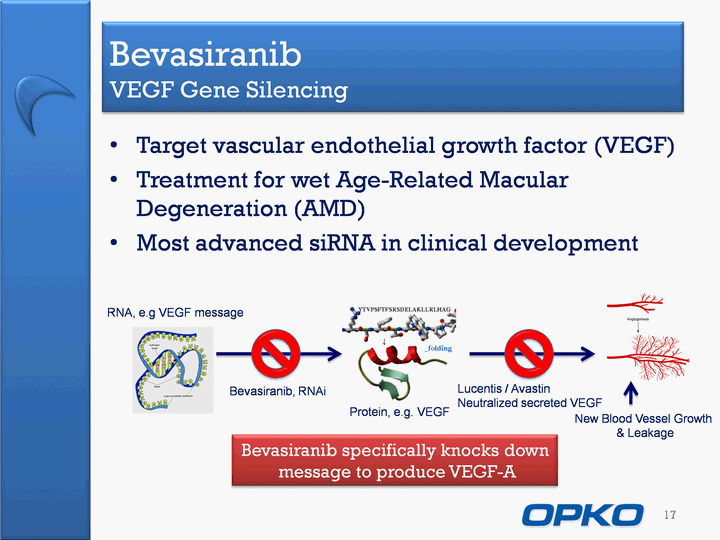
| Bevasiranib VEGF Gene Silencing Target vascular endothelial growth factor (VEGF)Treatment for wet Age-Related Macular Degeneration (AMD)Most advanced siRNA in clinical development 17 New Blood Vessel Growth & Leakage RNA, e.g VEGF message Protein, e.g. VEGF Lucentis / AvastinNeutralized secreted VEGF Bevasiranib, RNAi Bevasiranib specifically knocks down message to produce VEGF-A |
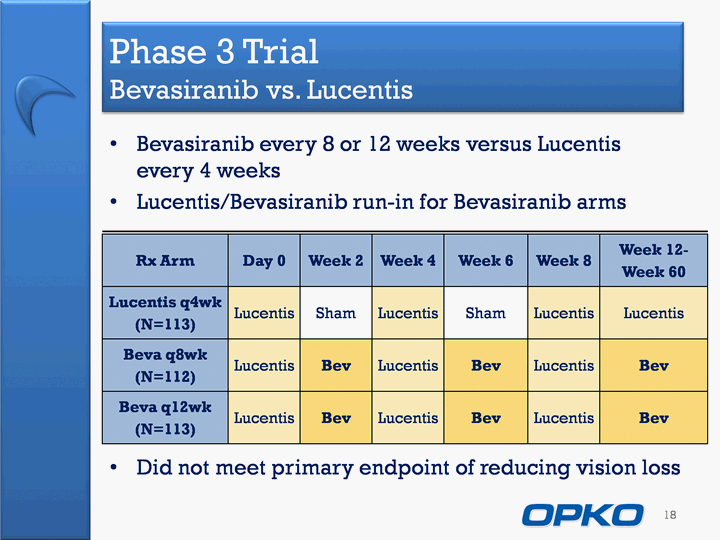
| Phase 3 Trial Bevasiranib vs. Lucentis Bevasiranib every 8 or 12 weeks versus Lucentis every 4 weeksLucentis/Bevasiranib run-in for Bevasiranib arms 18 Rx Arm Day 0 Week 2 Week 4 Week 6 Week 8 Week 12-Week 60 Lucentis q4wk(N=113) Lucentis Sham Lucentis Sham Lucentis Lucentis Beva q8wk(N=112) Lucentis Bev Lucentis Bev Lucentis Bev Beva q12wk(N=113) Lucentis Bev Lucentis Bev Lucentis Bev Did not meet primary endpoint of reducing vision loss |
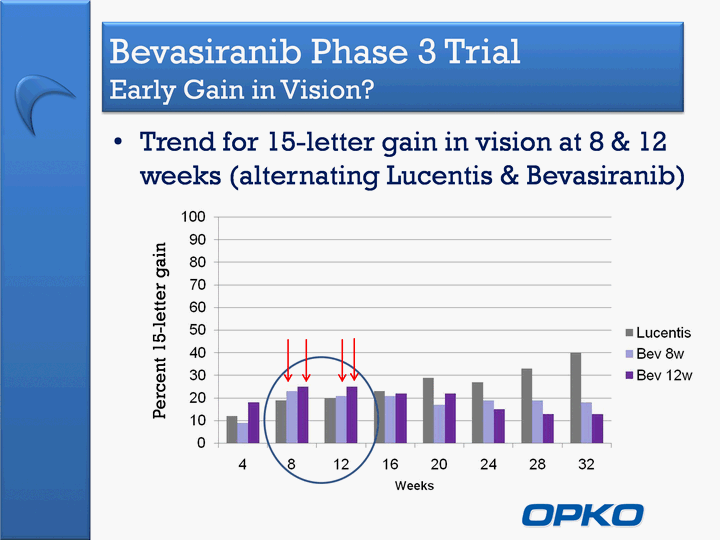
| Bevasiranib Phase 3 Trial Early Gain in Vision? Trend for 15-letter gain in vision at 8 & 12 weeks (alternating Lucentis & Bevasiranib) Percent 15-letter gain |
| Bevasiranib Next Steps Consider more frequent dosing Every 4 weeks like LucentisCombining with LucentisBased on promising activity during Lucentis run-in (up to 12 weeks)Reformulation with novel drug delivery vehicles (undisclosed)To enhance cellular penetration |
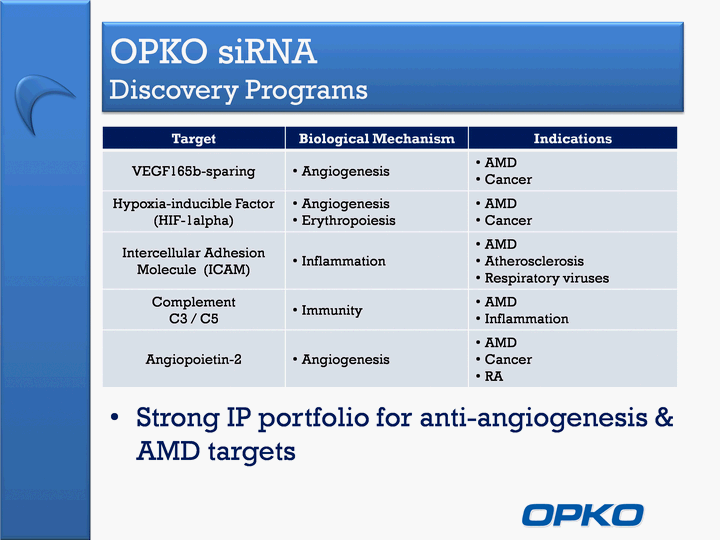
| OPKO siRNA Discovery Programs Target Biological Mechanism Indications VEGF165b-sparing Angiogenesis AMDCancer Hypoxia-inducible Factor (HIF-1alpha) AngiogenesisErythropoiesis AMDCancer Intercellular Adhesion Molecule (ICAM) Inflammation AMDAtherosclerosisRespiratory viruses Complement C3 / C5 Immunity AMDInflammation Angiopoietin-2 Angiogenesis AMDCancerRA Strong IP portfolio for anti-angiogenesis & AMD targets |

| Doxovir Viral Conjunctivitis Common eye infection, no effective treatmentDoxovir is a broad-spectrum anti-viral & anti-inflammatory agent with excellent preclinical efficacyEvaluated and shown to be safe in Phase I clinical trialsPhase 2 clinical trial to be initiated by OPKO in 3Q09 22 |

| Civamide Dry Eye Common eye illness with no effective treatmentCivamide is a villanoid receptor antagonist administered intranasallyInduces high frequency tearing in clinical trialsPhase 2 trial for dry eye to be initiated by OPKO in 3Q09 23 |
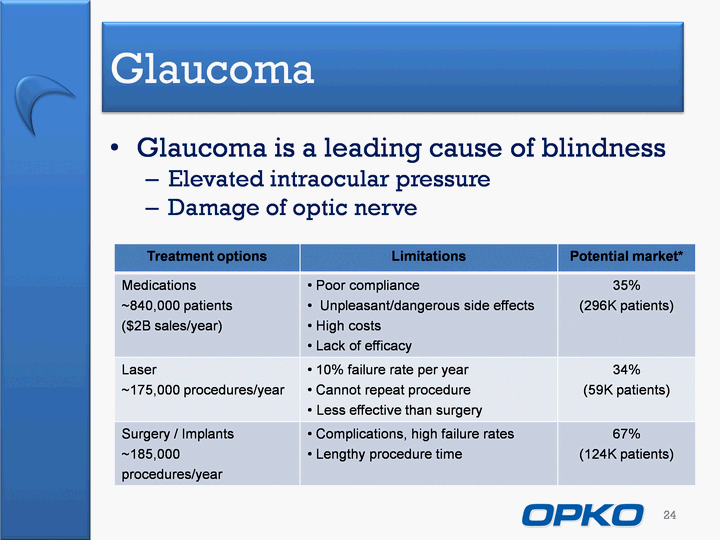
| Glaucoma Glaucoma is a leading cause of blindnessElevated intraocular pressureDamage of optic nerve 24 Treatment options Limitations Potential market* Medications~840,000 patients($2B sales/year) Poor compliance Unpleasant/dangerous side effects High costs Lack of efficacy 35%(296K patients) Laser~175,000 procedures/year 10% failure rate per year Cannot repeat procedureLess effective than surgery 34%(59K patients) Surgery / Implants~185,000procedures/year Complications, high failure rates Lengthy procedure time 67%(124K patients) |

| Aquashunt(tm) Glaucoma 25 "Tube vs Trab" study show shunts better than surgery Higher success rate Lower complication rateCurrent shunts can be improvedBetter efficacyLower complicationsLess complicated surgeryAquashunt(tm) is a novel shunt deviceSuprachoroidalSimple surgeryStrong IP |
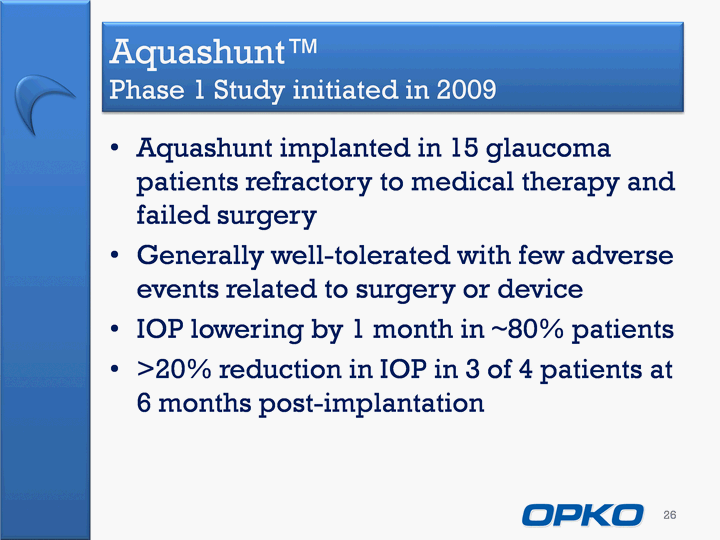
| Aquashunt(tm) Phase 1 Study initiated in 2009 Aquashunt implanted in 15 glaucoma patients refractory to medical therapy and failed surgeryGenerally well-tolerated with few adverse events related to surgery or deviceIOP lowering by 1 month in ~80% patients>20% reduction in IOP in 3 of 4 patients at 6 months post-implantation 26 |
| Influenza 200,000 hospitalizations and 46,000 deaths in U.S. each yearEconomic costs in U.S. ~$80 billionAnnual "flu shot" against common strains Flu vaccine grown in chicken eggsLimitations:New vaccine each yearOnly partially protectiveManufacturing capacity limited 27 |
| Universal Flu Vaccine Preclinical Development Universal flu vaccine licensed from Academia Sinica July 20, 2009Vaccine based on hemagglutinin to block viral entry Terminal sugars removed from hemagglutininCore common to all strains, including mutantsProtein-based vaccine more immunogenicPreclinical development initiated 28 |

| OPKO Future Expand applications and increase sales of ophthalmic instruments worldwideExplore innovative drug delivery systems for RNA interferenceDemonstrate clinical proof-of-concept with early clinical productsStrategic acquisitions of late stage clinical productsOpportunistic acquisitions of mature, profit- generating pharmaceutical businesses 29 |

| THANK YOU |