EX-99.2
Published on November 17, 2009
Exhibit 99.2

| Lazard Capital Markets 6th Annual Healthcare Conference November 17 - 18, 2009 New York, NY Jamie Freedman, MD, PhD Executive Vice President, R&D |

| Cautionary Statement 2 This presentation contains "forward-looking statements," as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA), which statements may be identified by words such as "expects," "plans," "projects," "will," "may," "anticipates," "believes," "should," "intends," "estimates," and other words of similar meaning, including statements regarding our ability to build a diverse portfolio of important medical products with significant commercial value, rapidly expand our drug development portfolio, our product development efforts and expected timing thereof, the products' potential benefits, statements regarding rolapitant being a best-in-class product, the timing of clinical trials for our product candidates and the commercial launch of rolapitant, estimates regarding market potential and timing of regulatory approval for our product candidates, our ability to invest in Research and Development, make strategic acquisitions of late stage clinical products and opportunistic acquisitions of mature profit- generating pharmaceutical businesses, and to make investments into high potential biotechnology companies, develop technologies for early detection of diseases such as Alzheimer's and cancer, as well as other non-historical statements. These forward-looking statements are only predictions and reflect our views as of the date they were made, and we undertake no obligation to update such statements. Such statements are subject to many risks and uncertainties that could cause our actual activities or results to differ materially from the activities and results anticipated in forward-looking statements, including risks inherent in funding, developing and obtaining regulatory approvals of new, commercially-viable and competitive products and treatments, general market factors, competitive product development, product availability, federal and state regulations and legislation, the regulatory process for new products and indications, manufacturing issues that may arise, the possibility of infringing a third party's patents or other intellectual property rights, the uncertainty of obtaining patents covering our products and processes and in successfully enforcing them against third parties, and the possibility of litigation, among other factors. |

| OPKO Health, Inc. A specialty healthcare company building a diverse portfolio of important medical products with significant commercial value 3 |

| OPKO Summary Expanding portfolio of medical products through internal R&D and licensing/acquisitions Opportunistic acquisitions of mature, profit-generating pharmaceutical businesses Private investments into high potential biotechnology companies 4 |
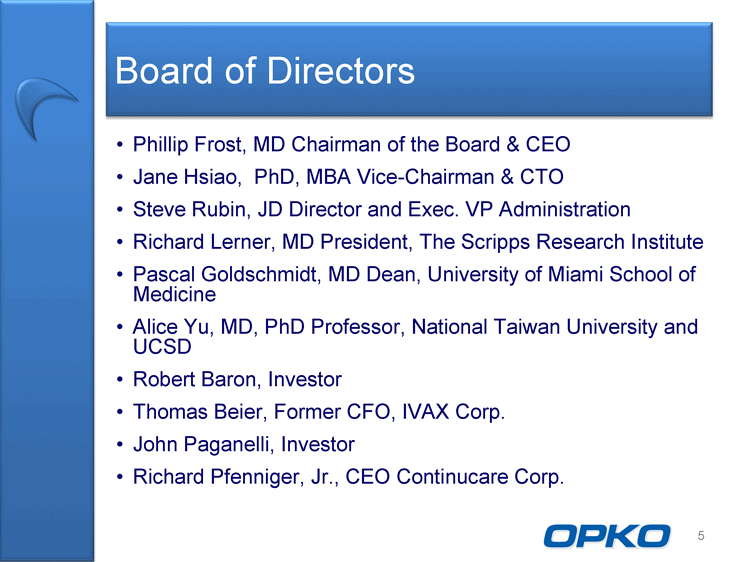
| Board of Directors Phillip Frost, MD Chairman of the Board & CEO Jane Hsiao, PhD, MBA Vice-Chairman & CTO Steve Rubin, JD Director and Exec. VP Administration Richard Lerner, MD President, The Scripps Research Institute Pascal Goldschmidt, MD Dean, University of Miami School of Medicine Alice Yu, MD, PhD Professor, National Taiwan University and UCSD Robert Baron, Investor Thomas Beier, Former CFO, IVAX Corp. John Paganelli, Investor Richard Pfenniger, Jr., CEO Continucare Corp. 5 |

| Management Team Phillip Frost, MD CEO & Chairman of the Board Jane Hsiao, PhD, MBA Vice-Chairman & Chief Technical Officer Steve Rubin, Exec. VP, Administration Rao Uppaluri, PhD, CFA Sr. VP & Chief Financial Officer Jamie Freedman, MD, PhD Exec. VP R&D 6 |
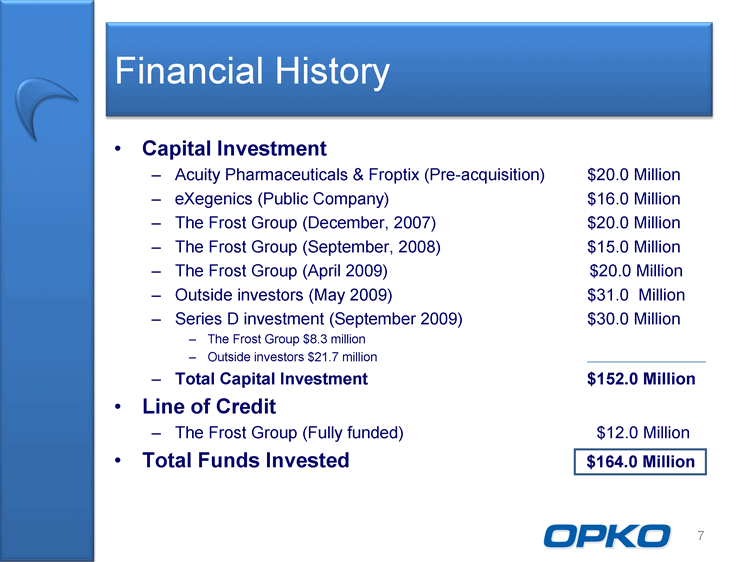
| Financial History Capital Investment Acuity Pharmaceuticals & Froptix (Pre-acquisition) $20.0 Million eXegenics (Public Company) $16.0 Million The Frost Group (December, 2007) $20.0 Million The Frost Group (September, 2008) $15.0 Million The Frost Group (April 2009) $20.0 Million Outside investors (May 2009) $31.0 Million Series D investment (September 2009) $30.0 Million The Frost Group $8.3 million Outside investors $21.7 million Total Capital Investment $152.0 Million Line of Credit The Frost Group (Fully funded) $12.0 Million Total Funds Invested $164.0 Million 7 |
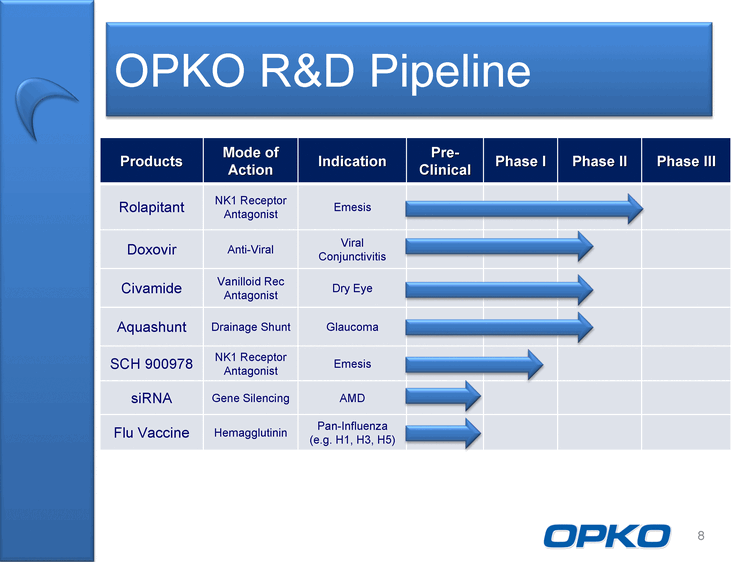
| OPKO R&D Pipeline 8 Products Mode of Action Indication Pre-Clinical Phase I Phase II Phase III Rolapitant NK1 Receptor Antagonist Emesis Doxovir Anti-Viral Viral Conjunctivitis Civamide Vanilloid Rec Antagonist Dry Eye Aquashunt Drainage Shunt Glaucoma SCH 900978 NK1 Receptor Antagonist Emesis siRNA Gene Silencing AMD Flu Vaccine Hemagglutinin Pan-Influenza (e.g. H1, H3, H5) |
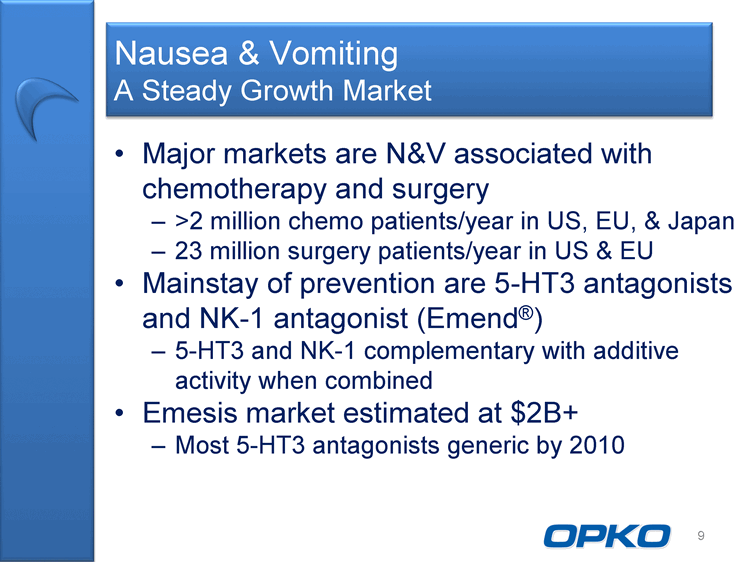
| Nausea & Vomiting A Steady Growth Market Major markets are N&V associated with chemotherapy and surgery >2 million chemo patients/year in US, EU, & Japan 23 million surgery patients/year in US & EU Mainstay of prevention are 5-HT3 antagonists and NK-1 antagonist (Emend(r)) 5-HT3 and NK-1 complementary with additive activity when combined Emesis market estimated at $2B+ Most 5-HT3 antagonists generic by 2010 9 |
| Rolapitant Potential Best-in-Class NK-1 Rolapitant acquired from Schering-Plough (Nov 2009) related to merger with Merck Phase 2 completed for chemotherapy and post-operative N&V (CINV/PONV) Oral and IV formulation in development Patent exclusivity for next 15+ years 10 |
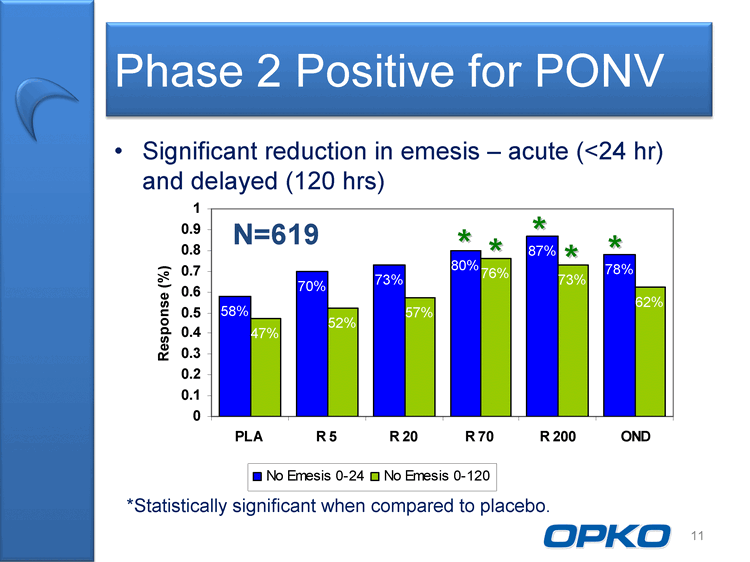
| Phase 2 Positive for PONV Significant reduction in emesis acute (24 hr) and delayed (120 hrs) 0.9 1 N=619 * * * * 58% 70% 73% 80% 87% 78% 52% 57% 76% 73% 62% 0.5 0.6 0.7 0.8 onse (%) * N 47% 0.1 0.2 0.3 0.4 Respo 0 PLA R 5 R 20 R 70 R 200 OND No Emesis 0-24 No Emesis 0-120 11 *Statistically significant when compared to placebo. OPKO La |
| Rolapitant Differentiation and Plans Key properties that make rolapitant profile better than competition ("best-in-class") No clinically significant drug-drug interactions Single dose regimen offering durable protection (5 days post dose) First to document sustained benefit of 5 day protection in PONV 1st indication PONV with potential launch in next 2-3 years 12 |
| Influenza 200,000 hospitalizations and 46,000 deaths in U.S. each year Economic costs in U.S. ~$80 billion a year Annual "flu shot" against common strains Flu vaccine grown in chicken eggs Limitations: New vaccine each year Only partially protective Manufacturing capacity limited 13 |
| Universal Flu Vaccine Preclinical Development Universal flu vaccine licensed from Academia Sinica July 2009 Vaccine based on modified hemagglutinin Broad protection against variants High potency Preclinical development initiated Large-scale manufacturing process being determined 14 |
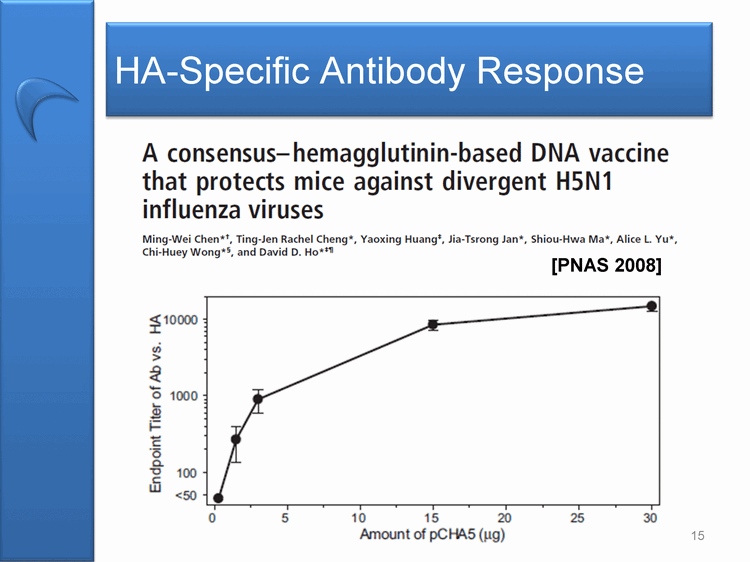
| HA-Specific Antibody Response A consensus-hemagglutinin-based DNA vaccine that protects mice against divergent H5N1 influenza viruses Ming-Wei Chen*+, Ting-Jen Rachel Cheng*, Yaoxing Huang+, Jia-Tsrong Jan*, Shiou-Hwa Ma*, ALice L. Yu*, Chi-Huey Wong*, and David D. Ho* 10000 1000 100 50 0 5 10 15 20 25 30 Amount of pCHA5(ug) 15 [PNAS 2008] |
| Molecular Diagnostics Overview Global molecular diagnostic market projected to reach ~$4B by 2010 Largest growth segments Early detection Companion diagnostics 16 |
| Molecular Diagnostics Small Molecule Microarray Molecular microarray technology acquired June 2009 "Peptoids" as first example of small molecule array to detect disease- associated antibodies Broad application for diagnosing diseases by a simple blood test Neurological disorders (Alzheimers Disease) Cancers (e.g. Lung, Pancreatic) Other Diseases 17 |
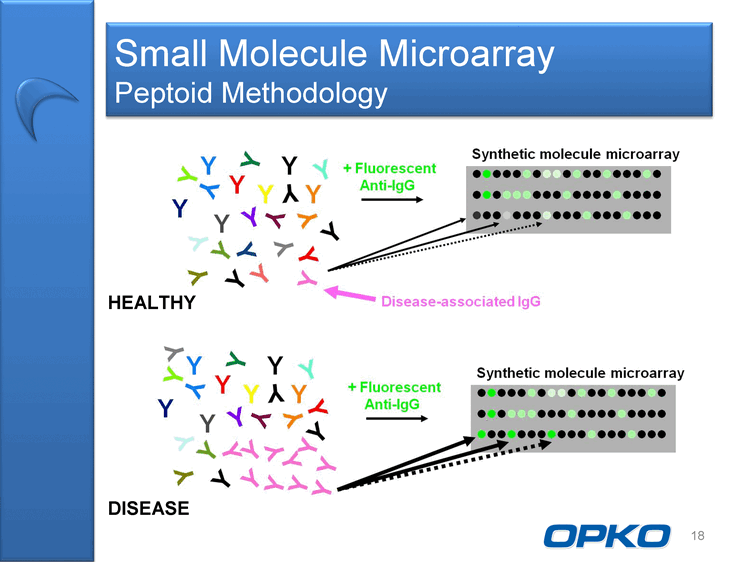
| Small Molecule Microarray Peptoid Methodology 18 HEALTHY DISEASE |
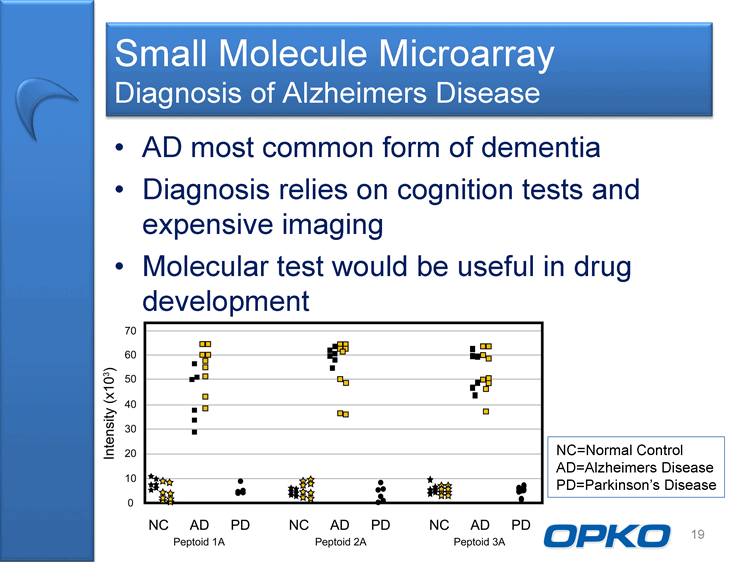
| Small Molecule Microarray Diagnosis of Alzheimers Disease AD most common form of dementia Diagnosis relies on cognition tests and expensive imaging Molecular test would be useful in drug development 19 NC=Normal Control AD=Alzheimers Disease PD=Parkinson's Disease |

| Multiple Peptoid Applications 20 Application Example Diagnostic Detection of disease and subpopulations Tissue-specific imaging agents Biomarkers to follow disease and response to therapy Therapeutic Identification of therapeutic targets Modulation of therapeutic targets (agonist or antagonist) Vaccine Antibody based vaccines T-cell based vaccines Dendritic cell therapy |
| Wide range of ultrasound products A-Scan, B-Scan, 3D-Scan, UBM Distributed in 45 countries Ultrasound market ~$60M a year OPKO Instruments Ultrasound 21 |
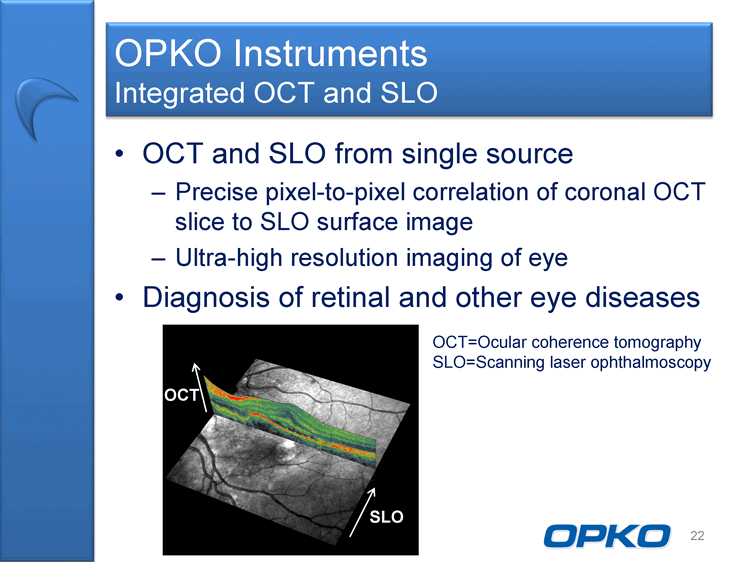
| OCT and SLO from single source Precise pixel-to-pixel correlation of coronal OCT slice to SLO surface image Ultra-high resolution imaging of eye Diagnosis of retinal and other eye diseases 22 OPKO Instruments Integrated OCT and SLO OCT SLO OCT=Ocular coherence tomography SLO=Scanning laser ophthalmoscopy |
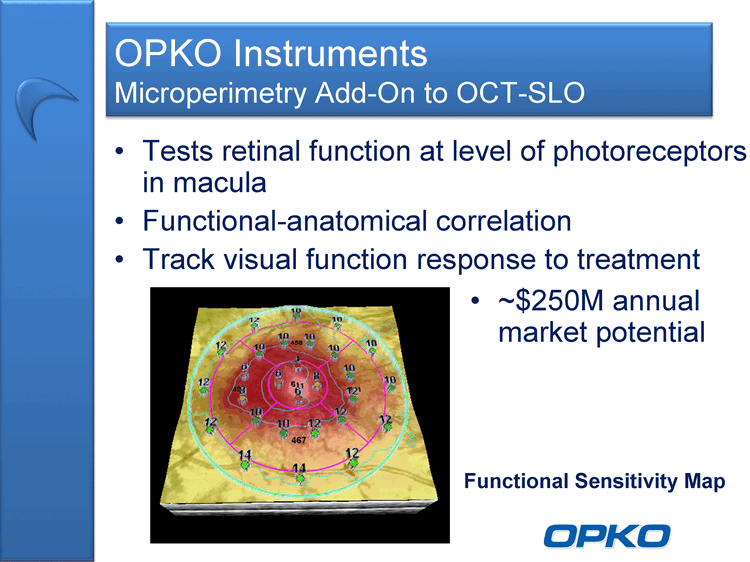
| OPKO Instruments Microperimetry Add-On to OCT-SLO Tests retinal function at level of photoreceptors in macula Functional-anatomical correlation Track visual function response to treatment Functional Sensitivity Map ~$250M annual market potential |

| Pharma Genexx Overview OPKO acquired Pharma Genexx (Chile) in Oct 2009 Rapidly growing sales and profits in Latin America Commercializing >90 products in multiple therapeutic areas 24 |
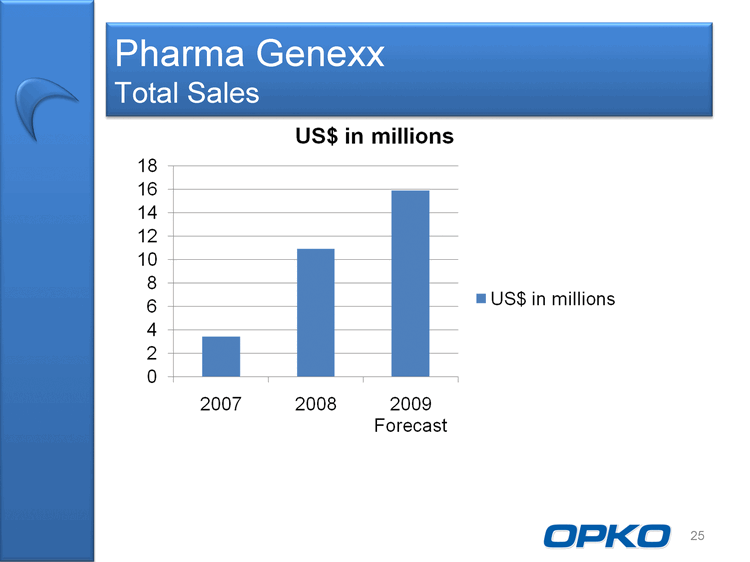
| Pharma Genexx Total Sales US$ in millions 0 2 4 6 8 10 12 14 16 18 2007 2008 2009 Forecast US$ in millions 25 |
| Minority Interest Platform for human monoclonal antibodies 26 Protein structure-based anti-viral therapeutics Broad spectrum CoCrystal Discovery Inc. |

| OPKO Summary Rapid expansion of drug development portfolio, including late stage compounds Profitable pharmaceutical companies acquired Minority interest in high potential biotechnology companies 27 |