EX-99.1
Published on June 1, 2010
Exhibit 99.1

| May 27, 2010 Annual Stockholders Meeting |

| Cautionary Statement This presentation contains "forward-looking statements," as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA), which statements may be identified by words such as "expects," "plans," "projects," "will," "may," "anticipates," "believes," "should," "intends," "estimates," and other words of similar meaning, including statements regarding our ability to develop oral and IV formulations of rolapitant and simple blood tests for neurological disorders, cancers and other disease, our product development efforts and expected timing thereof, the products' potential benefits, statements regarding rolapitant being a best-in-class product, the timing of clinical trials for our product candidates and the commercial launch of rolapitant and our other product candidates, and estimates regarding market potential and timing of regulatory approval for our product candidates, as well as other non-historical statements. These forward-looking statements are only predictions and reflect our views as of the date they were made, and we undertake no obligation to update such statements. Such statements are subject to many risks and uncertainties that could cause our actual activities or results to differ materially from the activities and results anticipated in forward-looking statements, including risks inherent in funding, developing and obtaining regulatory approvals of new, commercially-viable and competitive products and treatments, general market factors, competitive product development, product availability, federal and state regulations and legislation, the regulatory process for new products and indications, manufacturing issues that may arise, the possibility of infringing a third party's patents or other intellectual property rights, the uncertainty of obtaining patents covering our products and processes and in successfully enforcing them against third parties, and the possibility of litigation, among other factors. 2 |

| OPKO 2009 Highlights 2009 was year of investment and growth as OPKO expanded beyond ophthalmics into areas of major unmet medical need. Acquired two operating companies in Latin America Acquired several important products and technologies Raised $81 million in capital 3 |

| New Products & Technologies Acquired rolapitant and other NK-1 assets from Schering Plough. Phase II clinical testing completed for prevention of chemotherapy induced and post-operative induced nausea and vomiting. Company intends to pursue development for both indications 4 |

| New Products & Technologies (Cont'd.) Acquired rights to a platform technology used to create important new diagnostic tests, vaccines, and therapeutic agents. Presently working to develop simple blood tests for Alzheimer's disease, Parkinson's disease, lung cancer, and others. 5 |

| New Products & Technologies (Cont'd.) Acquired worldwide rights for new technology to develop vaccines against influenza and other viral infections. Acquired worldwide rights for new technology to target cancer cells and deliver chemotherapeutic agents to target site. 6 |

| Acquisitions and Investments Acquired Pharma Genexx, S.A. in October 2009. Sells broad range of products to private, hospital and institutional Markets in Chile. Acquired Pharmacos Exakta, S.A. de C.V. in February 2010. Manufactures, markets and sells broad range of ophthalmic and other pharmaceutical products in private and public markets in Mexico. 7 |
| Acquisitions and Investments Invested in Sorrento Therapeutics, Inc. 24% owned by OPKO. Became publicly traded in 2009. Recently announced validation of technology to produce larger human antibody libraries more efficiently than presently available methods. 8 |

| Acquisitions and Investments Invested in Cocrystal Discovery, Inc. 16% owned by OPKO. Privately held biopharmaceutical company Using novel approach to drug discovery to develop new broad spectrum anti-viral drugs. 9 |
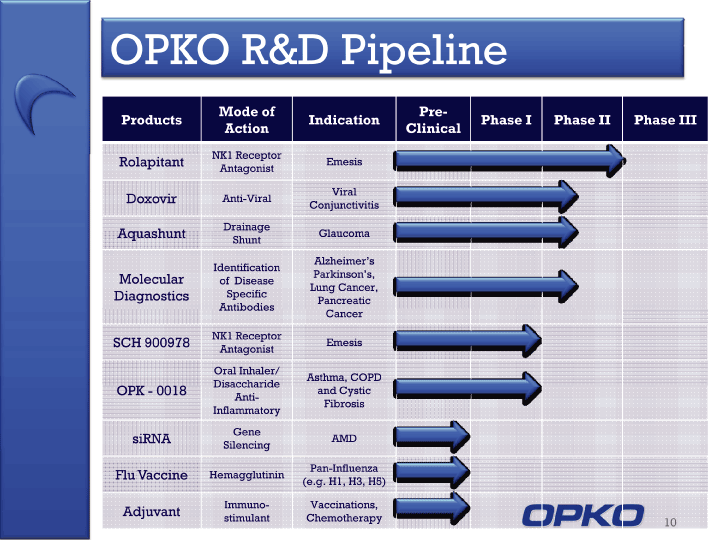
| OPKO R&D Pipeline 10 Products Mode of Action Indication Pre-Clinical Phase I Phase II Phase III Rolapitant NK1 Receptor Antagonist Emesis Doxovir Anti-Viral Viral Conjunctivitis Aquashunt Drainage Shunt Glaucoma Molecular Diagnostics Identification of Disease Specific Antibodies Alzheimer's Parkinson's, Lung Cancer, Pancreatic Cancer SCH 900978 NK1 Receptor Antagonist Emesis OPK - 0018 Oral Inhaler/ DisaccharideAnti-Inflammatory Asthma, COPD and Cystic Fibrosis siRNA Gene Silencing AMD Flu Vaccine Hemagglutinin Pan-Influenza (e.g. H1, H3, H5) Adjuvant Immuno-stimulant Vaccinations,Chemotherapy |
| Rolapitant Potential Best-in-Class NK-1 Receptor Antagonist Rolapitant acquired from Schering-Plough (Nov 2009) related to merger with Merck Phase 2 completed for chemotherapy and post-operative N&V (CINV/PONV) Successful end of Phase 2 meeting with FDA in April 2010 Oral and IV formulation in development Patent exclusivity for next 15+ years 11 |
| Rolapitant Differentiation and Plans Key properties that make rolapitant profile better than competition ("best-in-class") No clinically significant drug-drug interactions Single dose regimen offering durable protection (5 days post dose) First to document sustained benefit of 5 day protection in PONV 1st indication CINV with potential launch in next 2-3 years 12 |
| Influenza 200,000 hospitalizations and 46,000 deaths in U.S. each year Economic costs in U.S. ~$80 billion a year Annual "flu shot" against common strains Flu vaccine grown in chicken eggs Limitations: New vaccine each year Only partially protective Manufacturing capacity limited 13 |
| Universal Flu Vaccine Preclinical Development Universal flu vaccine licensed from Academia Sinica July 20, 2009 Vaccine based on modified hemagglutinin Broad protection against variants High potency Preclinical development initiated Large-scale manufacturing process being determined 14 |

| Adjuvant Small molecule adjuvant Improves the immune response in vaccines Enhances the pharmacological effect of chemotherapy agents Immunomodulator which suppresses tumors independently 15 |
| Molecular Diagnostics Overview Global molecular diagnostic market projected to reach ~$4B by 2010 Largest growth segments Early detection Companion diagnostics 16 |
| Molecular Diagnostics Small Molecule Microarray Molecular microarray technology acquired June 2009 "Peptoids" as first example of small molecule to detect disease-associated antibodies Broad application for diagnosing diseases by a simple blood test Neurological disorders (Alzheimer's Disease, Parkinson's) Cancers (e.g. Lung, Pancreatic) Other Diseases 17 |
| Asthma & COPD Over 22 million in the U.S. live with asthma, including more than 6 million children There are more than 11 million in the U.S. who have COPD Various drug treatments are available, but often with unwanted side effects and/or limited effectiveness Asthma & COPD Global Market was approximately U.S. $28 billion in 2007 18 |

| Lead Compound - OPK -0018 Novel Heparin-Derived Oligosaccharide Anti-Inflammatory Agent Heparin is a polysaccharide discovered in 1927 as an anticoagulant Heparin has been shown to have anti-inflammatory actions in a range of human disease but its anti-coagulant actions limit its use Multiple patents issued claiming various oligosaccharides and disaccharides and additional patents pending claiming specific formulations and uses In vivo anti-inflammatory/anti-allergic efficacy demonstrated in sheep and mice Effective orally inhaled with inhaler or nebulizer Phase 1 trials of two, single dose aerosol safety studies completed 19 |
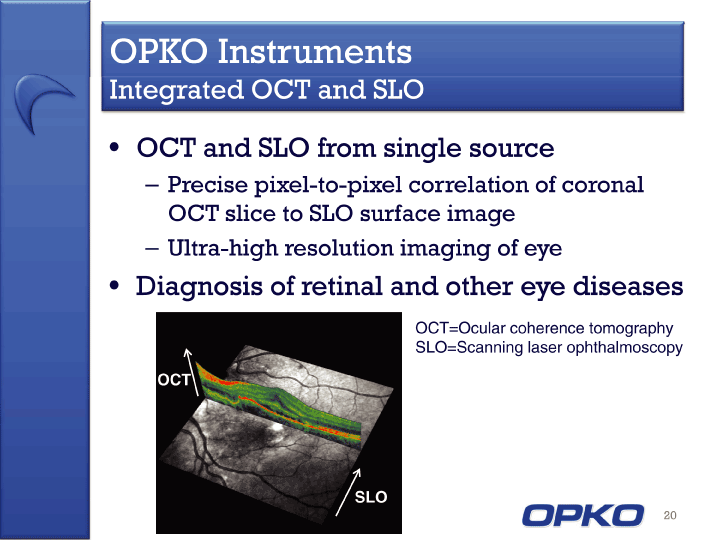
| OCT and SLO from single source Precise pixel-to-pixel correlation of coronal OCT slice to SLO surface image Ultra-high resolution imaging of eye Diagnosis of retinal and other eye diseases 20 OPKO Instruments Integrated OCT and SLO OCT SLO OCT=Ocular coherence tomography SLO=Scanning laser ophthalmoscopy |

| OPKO Summary Rapid expansion of drug development portfolio, including late stage compounds Profitable pharmaceutical companies acquired Minority interest in high potential biotechnology companies 21 |