EX-99.1
Published on June 10, 2011
Exhibit 99.1

| Annual Stockholders Meeting June 9, 2011 |

| Cautionary Statement This presentation contains "forward-looking statements," as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA), which statements may be identified by words such as "expects," "plans," "projects," "will," "may," "anticipates," "believes," "should," "intends," "estimates," "potential" and other words of similar meaning, including statements regarding our ability achieve high levels of growth, the potential for our products under development to be game- changing, our ability to develop additional diagnostics, our ability to develop certain therapeutics, our ability to develop, test and launch new products, the expected timing of the tests and trials relating to our products under development and product candidates, the expected size of the market for certain of our products under development, the potential benefits of our products under development, our ability to out-license certain of our technologies, our ability to successfully develop our product candidates such as simple blood tests for Alzheimer's disease, neurological disorders, cancers and autoimmune diseases and expected timing thereof, the products' potential benefits, statements regarding rolapitant being a best-in-class product, the timing of clinical trials for our product candidates and the commercial launch of rolapitant for CINV and our other product candidates, and estimates regarding market potential and timing of regulatory approval and potential launch dates for our product candidates, as well as other non-historical statements. These forward-looking statements are only predictions and reflect our views as of the date they were made, and we undertake no obligation to update such statements. Such statements are subject to many risks and uncertainties that could cause our activities or actual results to differ materially from the activities and results anticipated in forward-looking statements, including risks inherent in funding, developing and obtaining regulatory approvals of new, commercially-viable and competitive products and treatments, general market factors, competitive product development, product availability, federal and state regulations and legislation, delays associated with development of novel technologies, unexpected difficulties and delays in validating and testing product candidates, the regulatory process for new products and indications, manufacturing issues that may arise, the cost of funding lengthy research programs, the need for and availability of additional capital, the possibility of infringing a third party's patents or other intellectual property rights, the uncertainty of obtaining patents covering our products and processes and in successfully enforcing them against third parties, and the possibility of litigation, among other factors, including all of the risks identified under the heading Risk Factors in our Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. 2 |

| Management Team Phillip Frost, M.D. Chairman and CEO TEVA (Chairman), IVAX (Chairman and CEO), Key Pharmaceuticals (Chairman) Jane H. Hsiao, Ph.D. Vice Chairman and Chief Technology Officer IVAX (Chief Technology Officer) Steven Rubin Executive Vice President and Chief Administrative Officer IVAX (General Counsel) Rao Uppaluri, Ph.D. Senior Vice President and CFO IVAX (Treasurer) Tom Kodadek, Ph.D. Professor of Chemistry and Cancer Biology, Scripps Florida Research Institute Director of Chemistry and Molecular Biology at OPKO 3 |

| Company Overview Discovery Earlier Clinical Commercial Mid / Late Clinical Opportunistic pharmaceutical and diagnostics company Focused on large, high growth markets Experience in development and commercialization. Multiple game-changing opportunities New molecular diagnostics platform targeting conditions with unmet diagnostic needs (cancer, neurodegenerative diseases, etc.) New therapeutic technology (CURNA) based on gene up- regulation, initially targeting genetic diseases Next generation influenza vaccine (universal?) New drugs for asthma, COPD and cystic fibrosis Emerging markets pharmaceutical business Cash and cash equivalents of approximately $108 million as of 3/31/11 Net cash used for operations - $4.7 million for the 3 months ended 3/31/11 4 |
| Molecular Diagnostics Innovative platform technology for the discovery of disease-specific antibodies Initially targeting diseases for which there is no definitive blood test Simple tests for Alzheimer's Disease, multiple forms of cancer and other conditions Non-exclusive collaboration with BMS for lead Alzheimer's test Technology permits development of new drugs and vaccines Ongoing trials to support commercialization 5 |
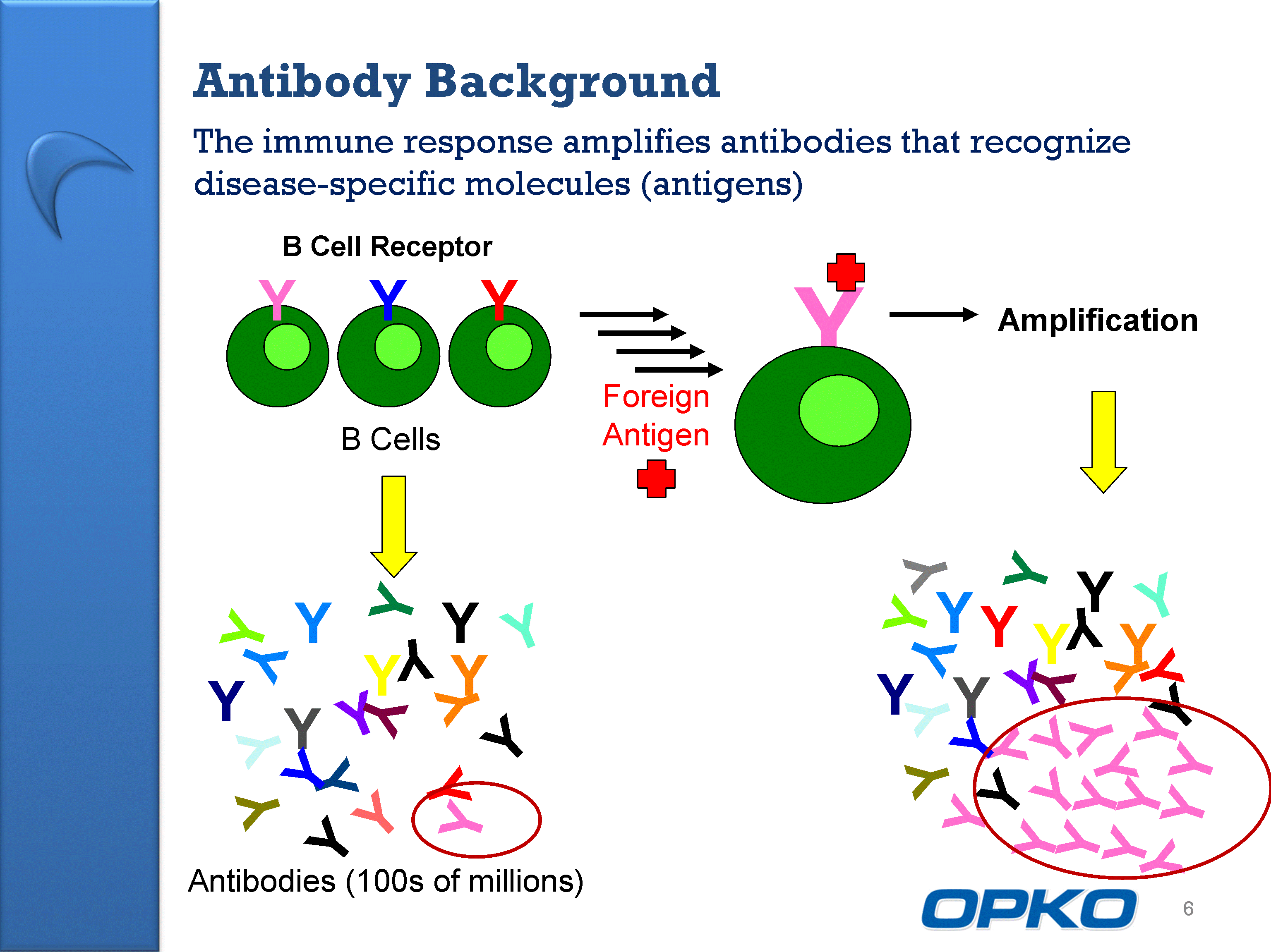
| The immune response amplifies antibodies that recognize disease-specific molecules (antigens) Antibody Background 6 Foreign Antigen Y Amplification Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y B Cells Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Antibodies (100s of millions) B Cell Receptor |
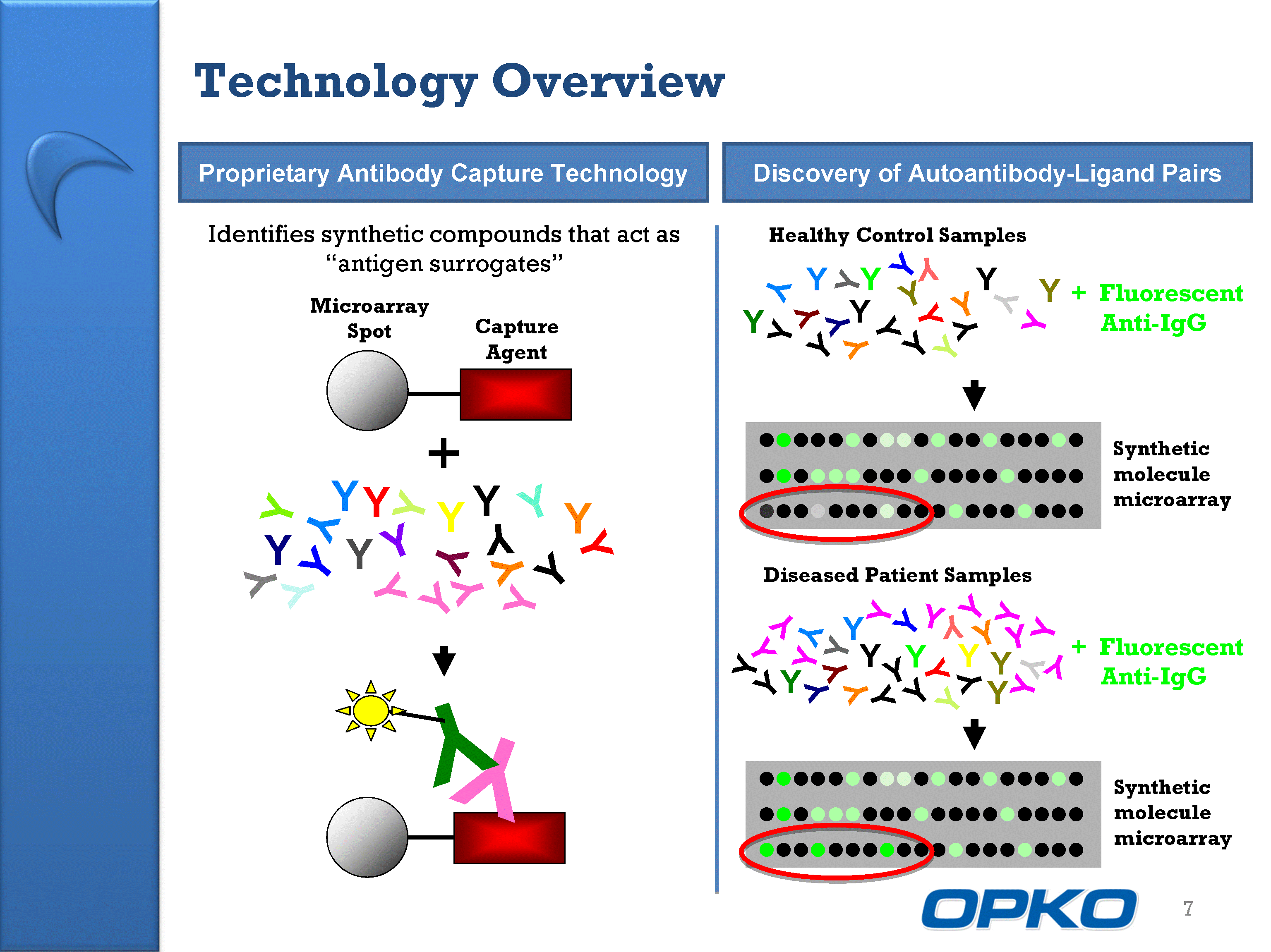
| Technology Overview 7 Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y Y + Y Y Identifies synthetic compounds that act as "antigen surrogates" Capture Agent Microarray Spot Proprietary Antibody Capture Technology Discovery of Autoantibody-Ligand Pairs Synthetic molecule microarray Healthy Control Samples Synthetic molecule microarray + Fluorescent Anti-IgG Diseased Patient Samples |
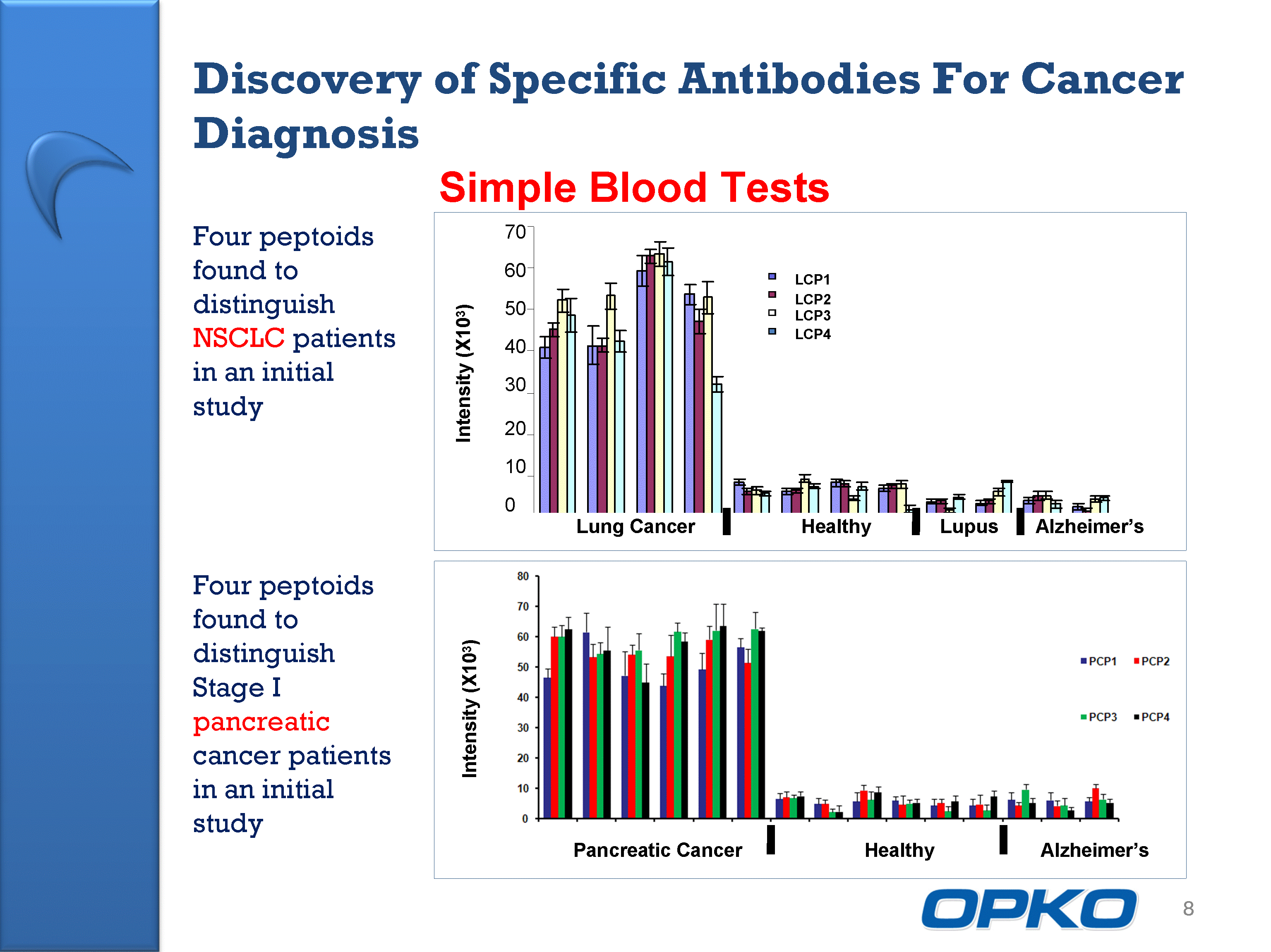
| 8 Four peptoids found to distinguish NSCLC patients in an initial study Four peptoids found to distinguish Stage I pancreatic cancer patients in an initial study Simple Blood Tests Discovery of Specific Antibodies For Cancer Diagnosis |
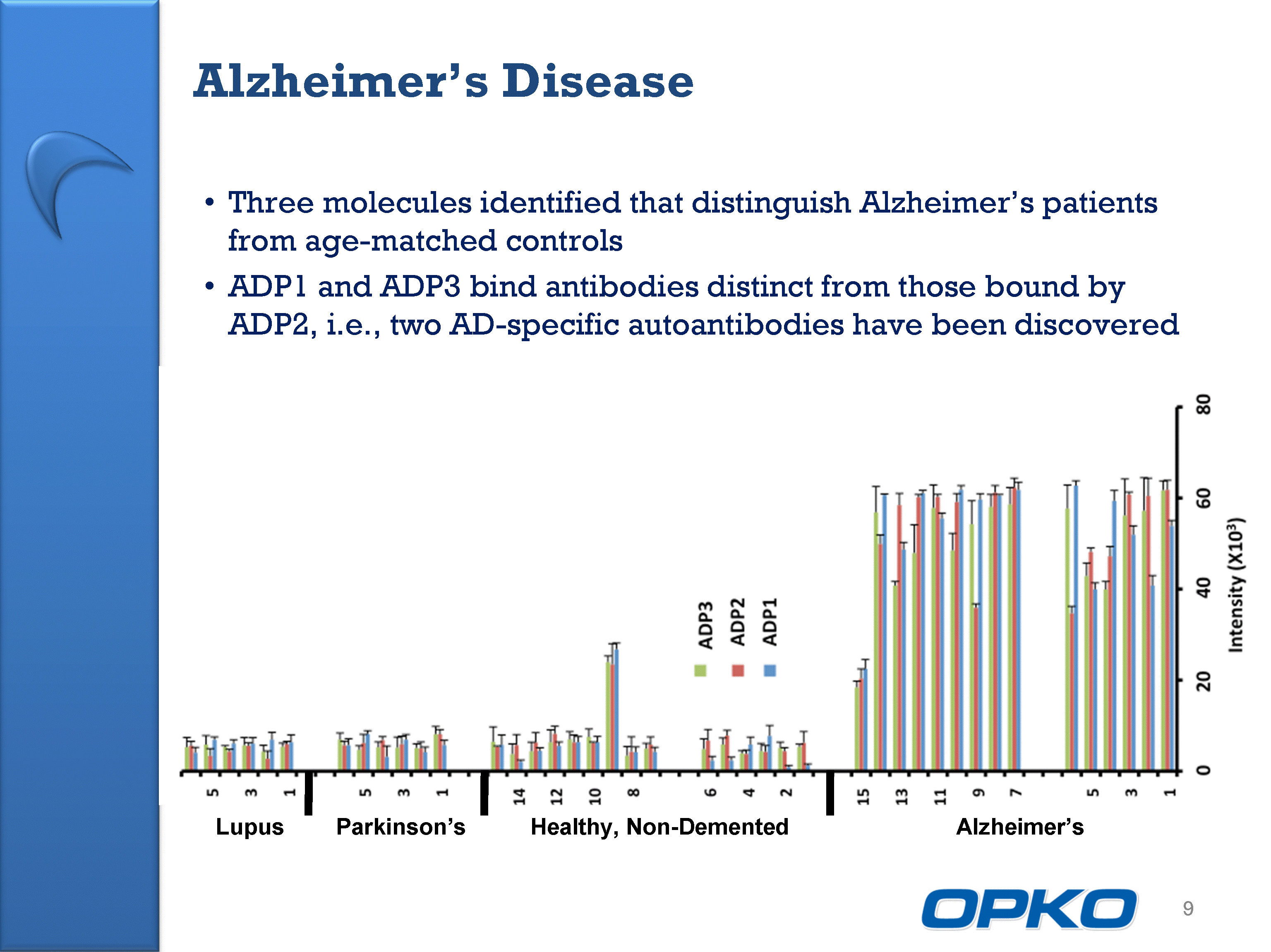
| Lupus Parkinson's Healthy, Non-Demented Alzheimer's Three molecules identified that distinguish Alzheimer's patients from age-matched controls ADP1 and ADP3 bind antibodies distinct from those bound by ADP2, i.e., two AD-specific autoantibodies have been discovered 9 Alzheimer's Disease |

| Alzheimer's Disease - Further Validation Conducting 580 patient sample validation study 200 AD, 200 matched controls, 180 other diseases Final results expected by late 2011 10 Ongoing validation study with positive initial results (140 samples) ADP1 Intensity ADP2 ADP3 Intensity Intensity |
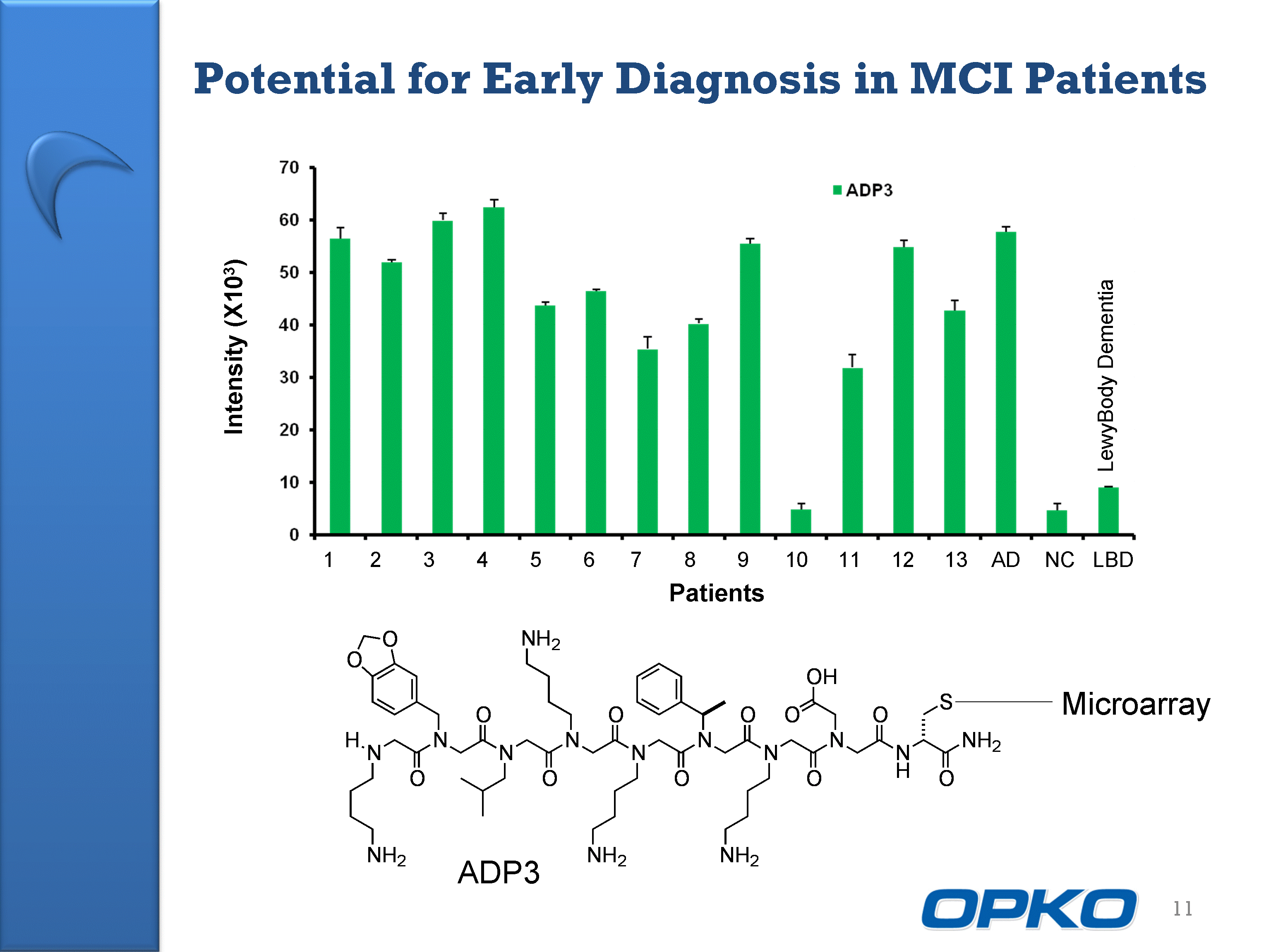
| 11 1 2 3 4 5 6 7 8 9 10 11 12 13 AD NC LBD LewyBody Dementia Patients Intensity (X103) Potential for Early Diagnosis in MCI Patients ADP3 Microarray |
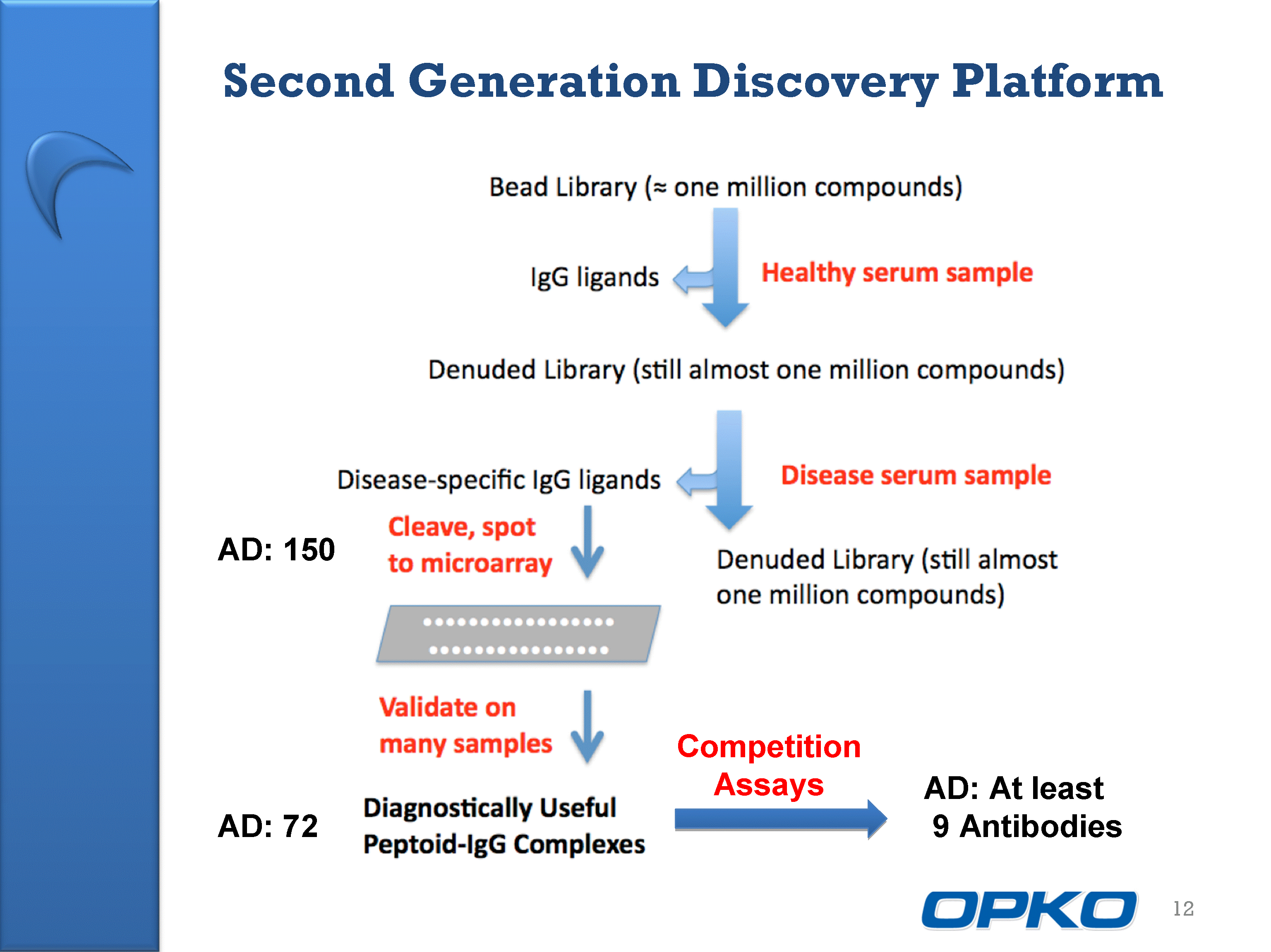
| Second Generation Discovery Platform 12 AD: 150 AD: 72 Competition Assays AD: At least 9 Antibodies |
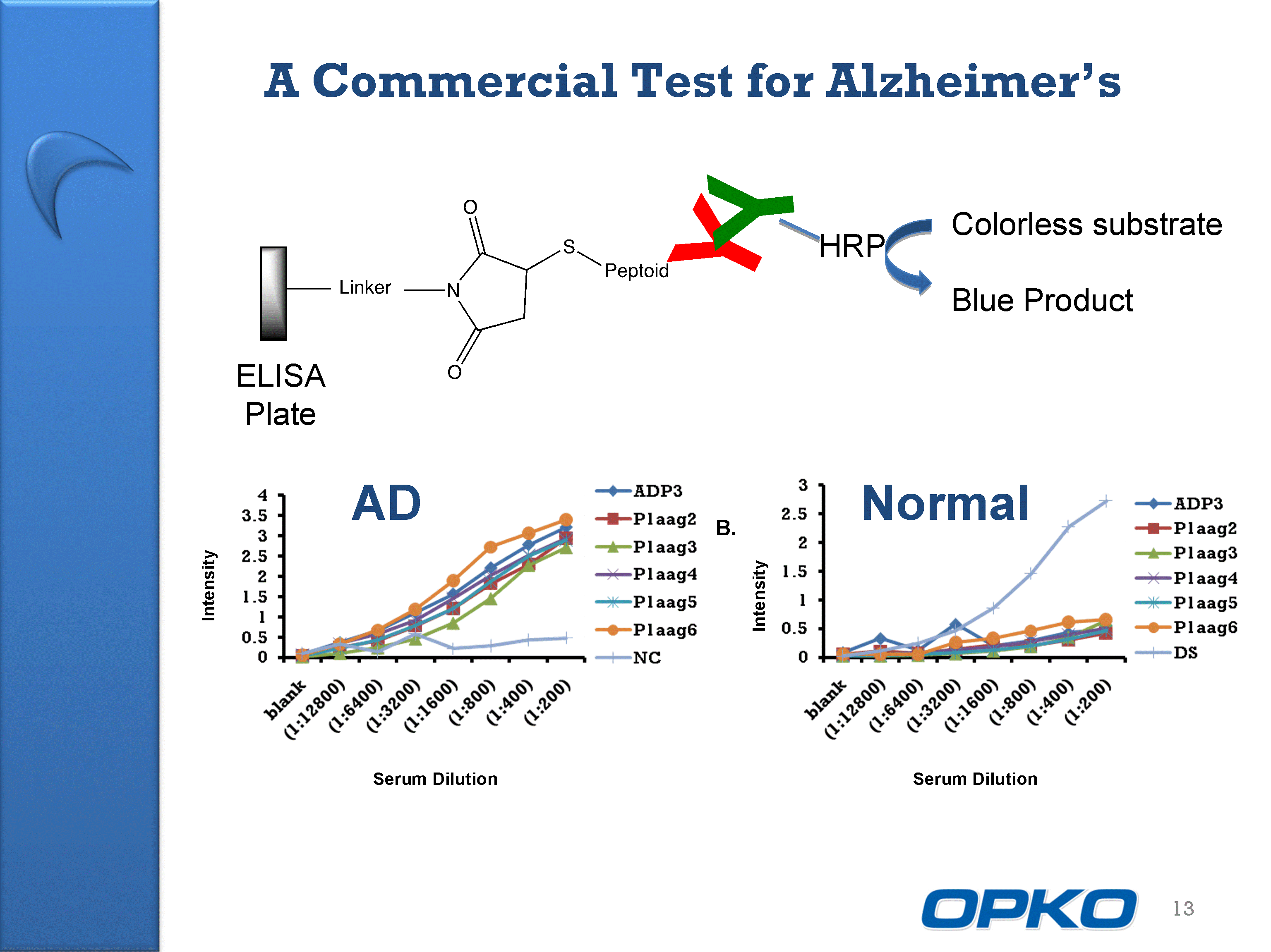
| A Commercial Test for Alzheimer's 13 Y Y HRP Colorless substrate Blue Product Serum Dilution Intensity AD Intensity Serum Dilution Normal B. ELISA Plate |
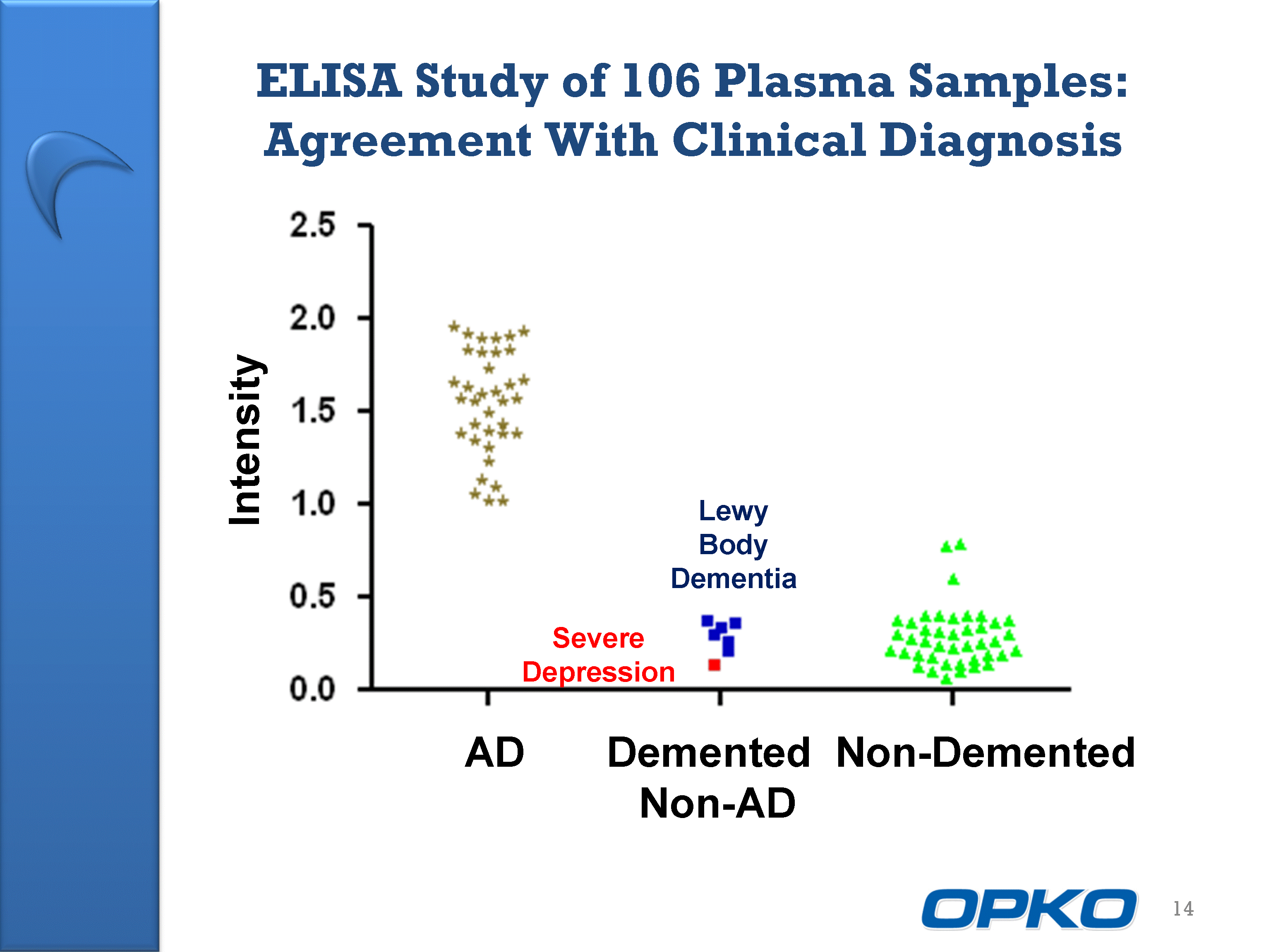
| ELISA Study of 106 Plasma Samples: Agreement With Clinical Diagnosis 14 Intensity AD Demented Non-Demented Non-AD Severe Depression Lewy Body Dementia |
| Summary of Diagnostic Work A highly reliable, commercially viable blood test for Alzheimer's disease is in hand. Test can distinguish between AD and certain other dementias. Preliminary results indicate high sensitivity for early stage Alzheimer's disease (MCI stage). R&D will be completed late summer 2011; planning for product launch. Lung and pancreatic cancer markers undergoing validation trials. R&D complete Q4 2012. Powerful, second generation discovery technology developed. 15 |

| Potential for Therapeutic Applications 16 Compound Library Therapeutic proof of concept demonstrated Initial proof of concept demonstrated in Pemphigus Vulgaris, an autoimmune skin blistering disease Approach: Screen for "desmoglein surrogates" that bind to the offending antibody and prevent it from attacking desmoglein Desmoglein Epidermis Dermis Separation of Skin Layers Desmoglein Surrogate |

| CURNA - Natural Antisense Transcript Inhibitors 17 Novel technology utilizes short, single-strand oligonucleotide Up-regulation of protein production through interference with non-coding RNA's (natural antisense) >80 protein targets across broad therapeutic spectrum were up-regulated in vitro Genetic diseases, cancers, neurological diseases Intravenous or subcutaneous delivery without complications of double-stranded, down-regulating siRNA therapeutics Encouraging animal study results to date Identifying targets to progress into clinical development May opportunistically out-license additional targets |
| Protein Vaccine Platform Limitations of current influenza vaccines Inability to respond to mutations ? inadequate protection Inefficient seasonal development and production timelines Minimally glycosylated protein vaccine provides broad protection against various viral strains Recombinant protein enables faster development and efficient year-round and demand-based production Global seasonal influenza market projected to be >$6bn by 2014 18 |

| Respiratory Therapeutics 19 OPK-0018: Lead Compound for Asthma & COPD Novel heparin-derived sulfated disaccharide Novel mechanism of action No anti-coagulant effects Human feasibility study for asthma successfully completed Anti-inflammatory and anti-allergic efficacy in animal models Effective orally or via inhalers/nebulizers Asthma /COPD market estimated $26 billion in 2009 with 34mm patients SCH 900978: NK-1 Receptor Antagonist for Chronic Cough Phase II study completed No safety issues identified Patent exclusivity for next 16+ years |
| Rolapitant - Phase III (out-licensed) 20 Potential best-in-class compound for CINV NK-1 Receptor Antagonist for CINV and PONV Acquired from Schering-Plough and out-licensed to TESARO Up to $121mm milestones plus tiered double-digit royalties Global emesis market estimated at $2B+ in 2009 Positive Phase II studies completed Long duration of action - single dose provides 5 days activity No significant drug-drug interactions Potential launch in 2-3 yrs Patent exclusivity for next 15+ years Tesaro co-founded by former executives of MGI Pharma Led successful U.S. commercialization of Aloxi for CINV Developed into market-leading 5-HT3 receptor antagonist Significant development and commercialization experience |
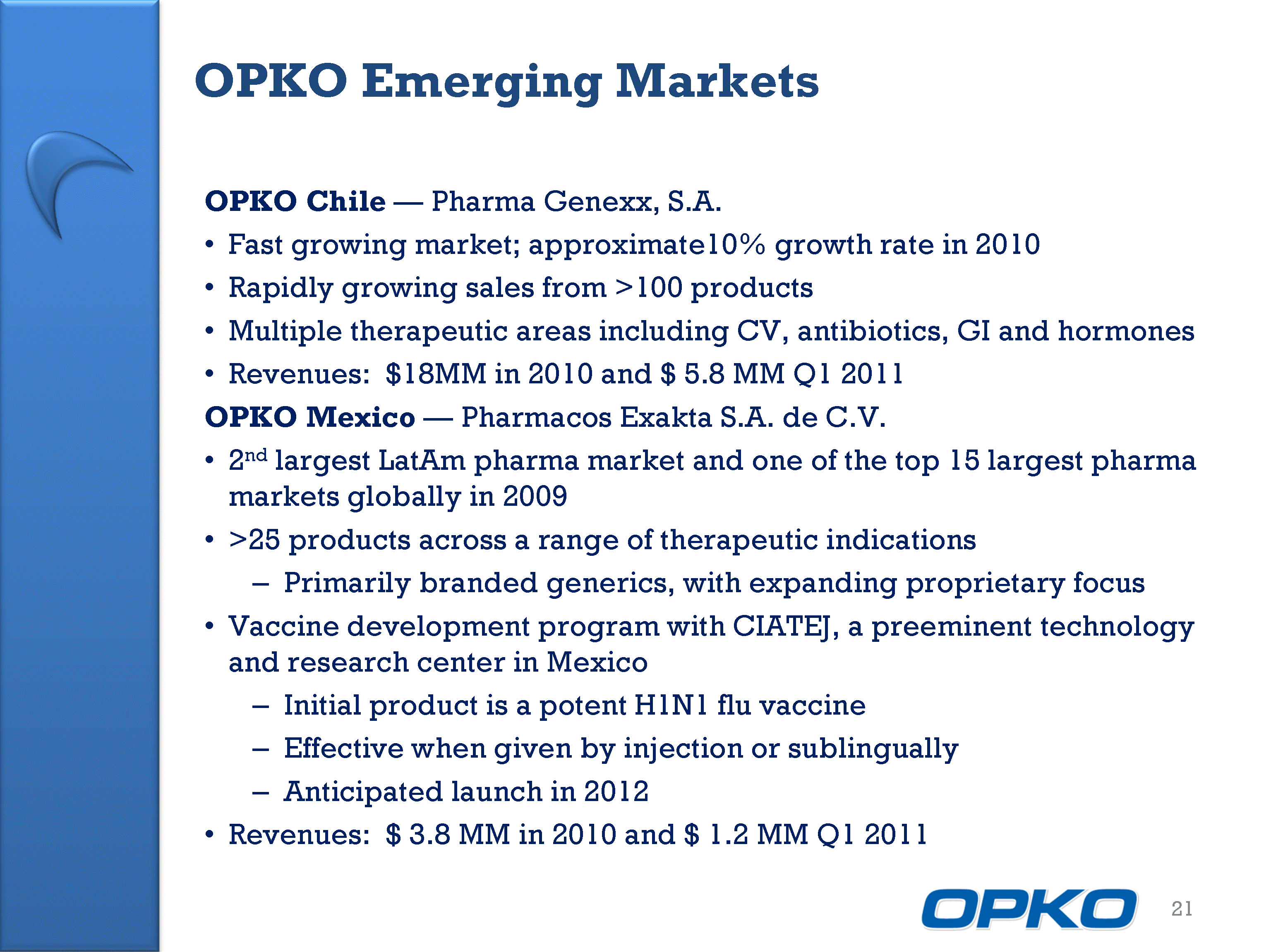
| OPKO Emerging Markets 21 OPKO Chile - Pharma Genexx, S.A. Fast growing market; approximate10% growth rate in 2010 Rapidly growing sales from >100 products Multiple therapeutic areas including CV, antibiotics, GI and hormones Revenues: $18MM in 2010 and $ 5.8 MM Q1 2011 OPKO Mexico - Pharmacos Exakta S.A. de C.V. 2nd largest LatAm pharma market and one of the top 15 largest pharma markets globally in 2009 >25 products across a range of therapeutic indications Primarily branded generics, with expanding proprietary focus Vaccine development program with CIATEJ, a preeminent technology and research center in Mexico Initial product is a potent H1N1 flu vaccine Effective when given by injection or sublingually Anticipated launch in 2012 Revenues: $ 3.8 MM in 2010 and $ 1.2 MM Q1 2011 |

| Strategic Investments 22 CoCrystal Discovery, Inc. (~16% equity interest*) Founded by Nobel Laureate, Roger Kornberg, Ph.D. New approach to develop broad spectrum anti-viral drugs Sorrento Therapeutics (~23% equity interest*) New technology to produce human monoclonal antibodies libraries that are more complete and more efficient Fabrus, LLC (~13% equity interest*) Next gen technology to identify therapeutic antibody targets Founded by Vaughn Smider, M.D., Ph.D. Assistant Professor of Molecular Biology, The Scripps Research Institute Tesaro, Inc. (~6% equity interest*) Oncology-focused biopharmaceutical company Founded by former executives of MGI Pharm * As of March 31, 2011 Proprietary technologies with significant upside potential |
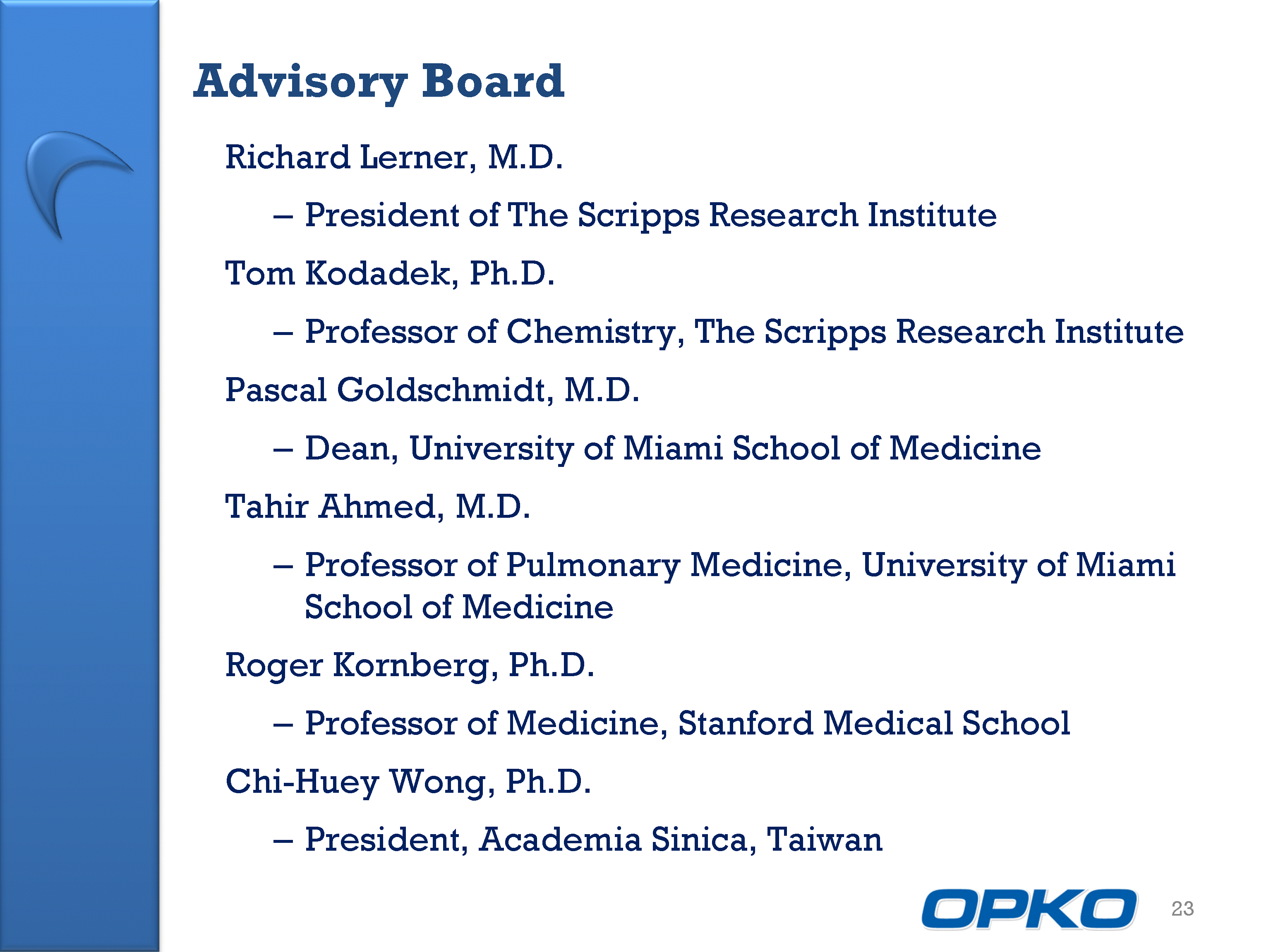
| Advisory Board Richard Lerner, M.D. President of The Scripps Research Institute Tom Kodadek, Ph.D. Professor of Chemistry, The Scripps Research Institute Pascal Goldschmidt, M.D. Dean, University of Miami School of Medicine Tahir Ahmed, M.D. Professor of Pulmonary Medicine, University of Miami School of Medicine Roger Kornberg, Ph.D. Professor of Medicine, Stanford Medical School Chi-Huey Wong, Ph.D. President, Academia Sinica, Taiwan 23 |