EX-99.1
Published on June 5, 2013
| Exhibit 99.1
|
Jefferies 2013 Global Healthcare Conference
June 4, 2013
|
|
This presentation contains forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA), which statements may be identified by words such as expects,plans,projects,will, may, anticipates, believes, should, intends, estimates, potential and other words of similar meaning, including statements regarding our estimated revenues and financial projections, our ability to achieve high levels of growth, the potential for Rayaldy, Fermagate tablets, Rolapitant, hGH-CTP and our other products in development, whether Rayaldy safety and efficacy results will drive untreated patients to start treatment with Rayaldy whether Rayaldy will take significant market share in the CKD Stage 3 and 4 markets, whether PROLORs hGH-CTP product will be the first long-acting growth hormone product to market, the potential for our next generation prostate markers to dramatically reduce biopsies and increase detection of cancer, our ability to develop certain therapeutics, new drugs and vaccines, including for the treatment of genetic diseases, cancers, neurological and metabolic disorders, our ability to develop, test and launch new products, the expected outcome and expected timing of the validation studies and clinical trials relating to our products under development, including without limitation, Rayaldy, Fermagate tablets, hGH-CTP, the 4KScore and certain diagnostic products, that such trials and studies will support commercialization, the expected market penetration and size of the market for certain of our products under development including rolapitant, Rayaldy and our diagnostics products, the potential benefits of our products under development, our ability to successfully develop and commercialize simple blood tests for Alzheimers, Multiple Sclerosis, Type I Diabetes, lung, pancreatic, and other cancers and autoimmune diseases, as well as diagnostic products for other markets such as urology, womens health, cardiology and infectious disease, our ability to develop and commercialize next generation prostate markers, and the timing of expected U.S. and European regulatory approvals for our pharmaceutical and diagnostic product candidates and the commercial launch of our pharmaceutical and diagnostic product candidates, as well as other non-historical statements. These forward-looking statements are only predictions and reflect our views as of the date they were made, and we undertake no obligation to update such statements. Such statements are subject to many risks and uncertainties that could cause our activities or actual results to differ materially from the activities and results anticipated in forward-looking statements, including risks inherent in funding, developing and obtaining regulatory approvals of new, commercially-viable and competitive products and treatments, general market factors, competitive product development, product availability, federal and state regulations and legislation, our ability to complete the acquisition of PROLOR and integration issues arising from the transaction, delays associated with development of novel technologies, unexpected difficulties and delays in validating and testing product candidates, the regulatory process for new products and indications, manufacturing issues that may arise, the cost of funding lengthy research programs, the need for and availability of additional capital, the possibility of infringing a third partys patents or other intellectual property rights, the uncertainty of obtaining patents covering our products and processes and in
successfully enforcing them against third parties, and the possibility of litigation, among other factors, including all of the risks identified under the heading Risk Factors in our Annual Report on Form 10-K and other filings with the Securities and Exchange Commission.
| 2 |
|
|
|
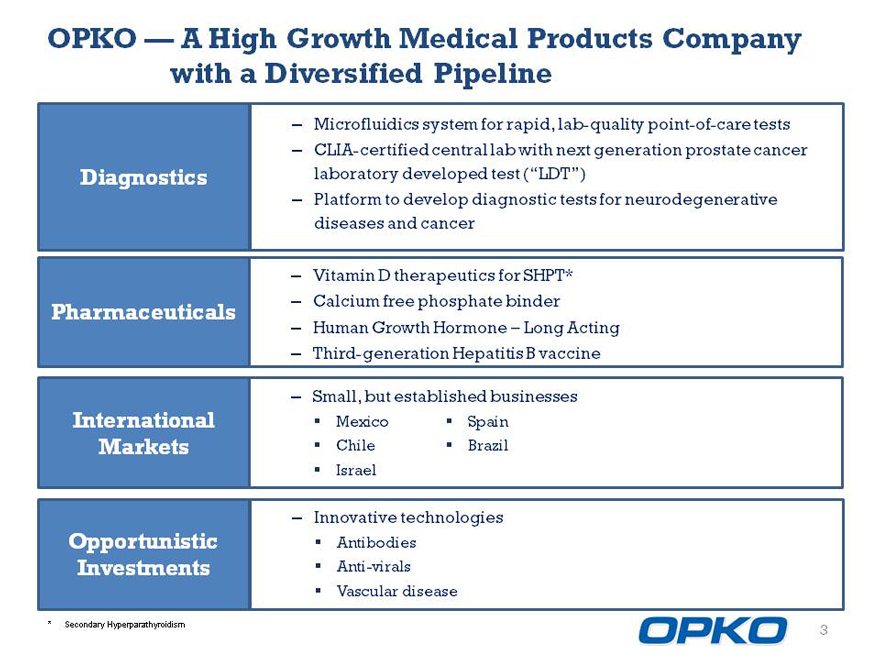
OPKO A High Growth Medical Products Company with a Diversified Pipeline
Microfluidics system for rapid, lab-quality point-of-care tests
CLIA-certified central lab with next generation prostate cancer laboratory developed test (LDT)
Platform to develop diagnostic tests for neurodegenerative diseases and cancer
Vitamin D therapeutics for SHPT*
Pharmaceuticals
International Mexico Spain
Markets Chile Brazil
Israel
Opportunistic Antibodies
Anti-virals
Vascular disease
| 3 |
|
|
|
Key Milestones in H1 2013
Phase 3 trials for RayaldyTM (CTAP101 Capsules) for treatment of SHPT continuing on schedule
top line results expected mid-2014
Entered into definitive agreement to acquire PROLOR BioTech
Issued $175 million aggregate principal amount of 3.00% convertible senior notes due 2033
Net cash position at $181.6 million as of March 31, 2013
Top-line Phase 3 data ata fo or r Rolapitant expected pected from Tesaro in H2 2013
Acquired interest in growing Russian pharmaceutical company
Entered into strategic pooling of assets with RXi Pharmaceuticals
Readying 4KScore commercial launch
| 4 |
|
|
|
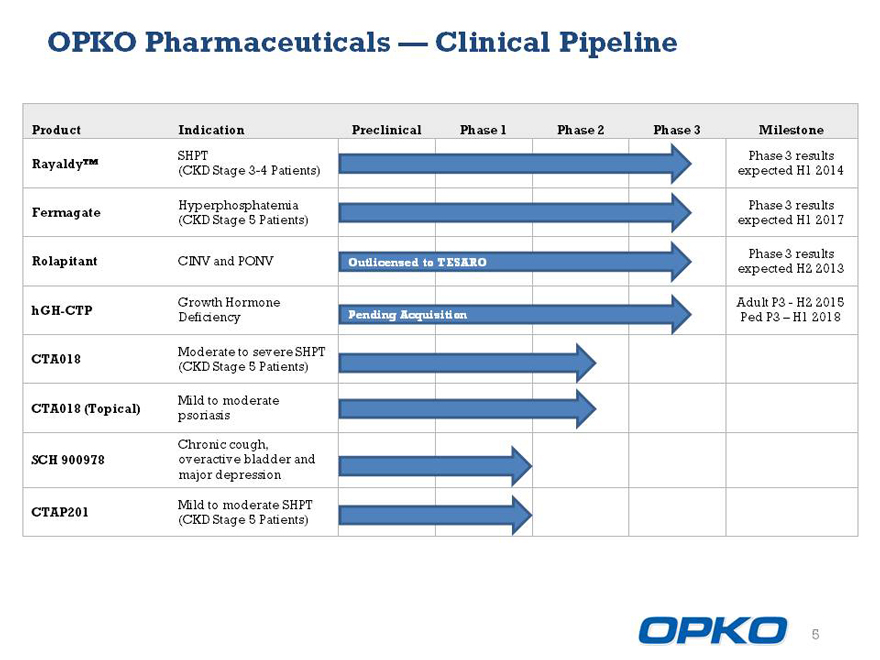
OPKO Pharmaceuticals Clinical Pipeline
Product Indication Preclinical Phase 1 Phase 2 Phase 3 Milestone
Phase 3 results
RayaldyTM
(CKD Stage 3-4 Patients) expected H1 2014
Fermagate expected H1 2017
Phase 3 results
Rolapitant CINV and PONV Outlicensed to TESARO expected H2 2013
hGH-CTP
Pending Acquisition H1 2018
CTA018
CTA018 (Topical) SCH 900978 CTAP201
| 5 |
|
|
|
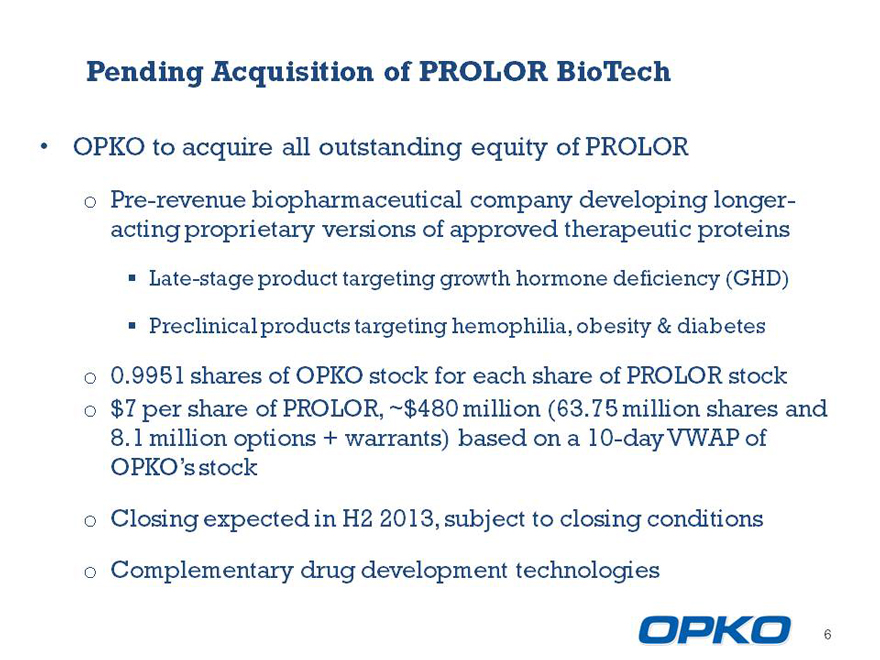
Pending Acquisition of PROLOR BioTech
OPKO to acquire all outstanding equity of PROLOR
o Pre-revenue biopharmaceutical company developing longer-acting proprietary versions of approved therapeutic proteins
Late-stage product targeting growth hormone deficiency (GHD)
Preclinical products targeting hemophilia, obesity & diabetes
o 0.9951 shares of OPKO stock for each share of PROLOR stock o $7 per share of PROLOR, ~$480 million (63.75 million shares and
8.1 million options + warrants) based on a 10-day VWAP of OPKOs stock
o Closing expected in H2 2013, subject to closing conditions
o Complementary drug development technologies
| 6 |
|
|
|
Effective hGH Therapy in Growth Hormone Deficient Adults
hGH-CTP is designed to:
Increase bone density Decrease fat mass
Increase lean body mass
Improve physical performance Improve cardiac function
| 7 |
|
|
|
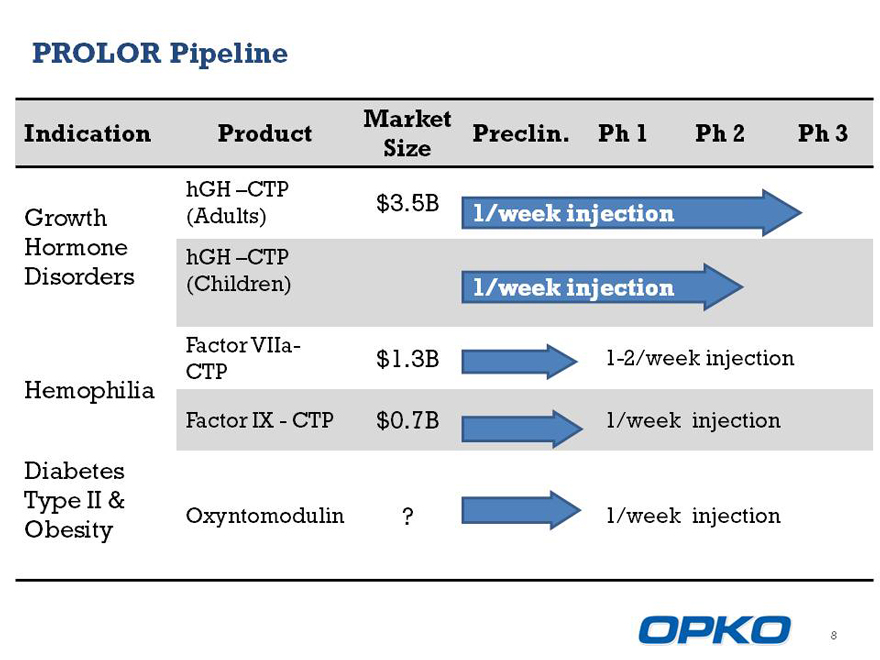
PROLOR Pipeline
Market
Indication Product Preclin. Ph 1 Ph 2 Ph 3 Size
hGH CTP
$3.5B
Growth (Adults) 1/week injection
Hormone hGH CTP
Disorders
Factor VIIa-
$1.3B 1-2/week injection CTP
Hemophilia
Factor IXCTP $0.7B 1/week injection
Diabetes
Type II & Oxyntomodulin ? 1/week injection
Obesity
| 8 |
|
|
|
PROLOR: hGH-CTP Clinical Program
189 patients, >40 centers in US, EU, Israel
12 months duration (6 months efficacy + 6 months safety)
Weekly injection compared to placebo control
Primary endpoint: Change in truncal fat mass after 6 months vs placebo
Ongoing Pediatric Phase 2 trial
40-56 naïve growth hormone deficient children
12 months efficacy & safety
Key outcome: Height velocity
Dose finding study 3 doses
Weekly injection vs. control group administered with daily commercial hGH
35 centers (EU, US, Israel)
Expected to be first long-acting growth hormone to market
Orphan drug designation in adults and pediatric GHD
9
|
|
Cytochroma Acquistion Product Highlights
First-in-class modified-release vitamin D prohormone that is designed to effectively control SHPT* in patients with Stage 3 or Stage 4 CKD and vitamin D insufficiency
Unmet Medical Need: No current therapy can reliably restore adequate serum 25D** and suppress elevated PTH*** while maintaining normal serum calcium levels
Compelling Phase 2b data in hand; Phase 3 program ongoing under Special Protocol Assessment
USPTO and EPO have issued or allowed patents covering the product until 2028
NDA filing expected in first half of 2015
Two Phase 2 and two Phase 3 clinical programs in CKD and psoriasis
Early stage pipeline includes new inhibitors of CYP24 and phosphate transport in the GI tract
Cytochromas product candidates address the current $2.3 billion market in the U.S. SHPT affects 50-60% of the 8 million CKD Stage 3-4 patients in the U.S.
| * |
|
SHPT = Secondary hyperparathyroidism |
** 25D = 25-hydroxyvitamin D 10 *** PTH = Parathyroid hormone
10
|
|
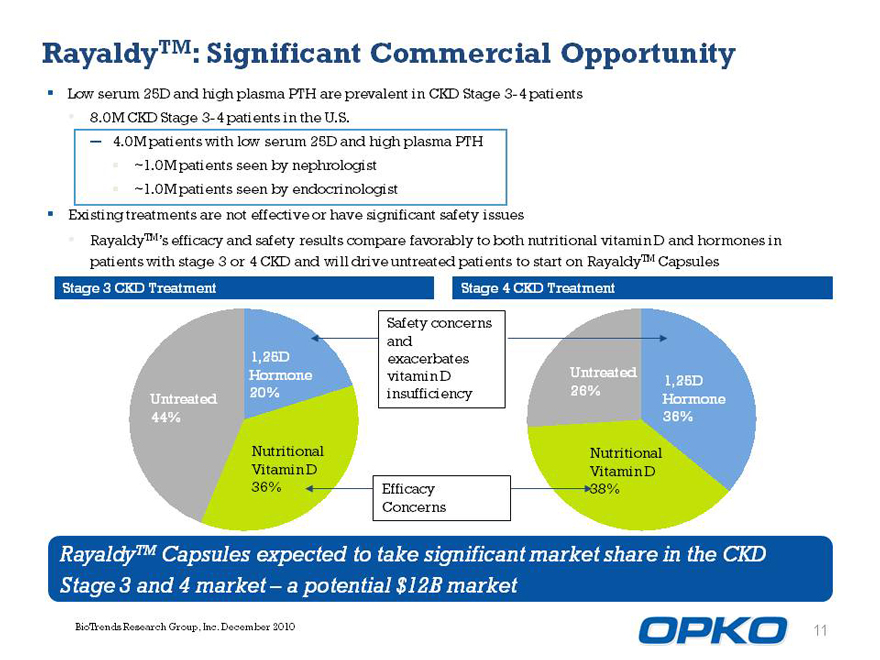
Rayaldytm: Siggnificant commercial opportunity
Low serum 25D and high plasma PTH are prevalent in CKD Stage 3-4 patients
8.0M CKD Stage 3-4 patients in the U.S.
? 4.0M patients with low serum 25D and high plasma PTH
~1.0M patients seen by nephrologist
~1.0M patients seen by endocrinologist
Existing treatments are not effective or have significant safety issues
RayaldyTMs efficacy and safety results compare favorably to both nutritional vitamin D and hormones in patients with stage 3 or 4 CKD and will drive untreated patients to start on RayaldyTM Capsules
Stage 3 CKD Treatment Stage 4 CKD Treatment
Safety concerns and 1,25D exacerbates
Hormone vitamin D Untreated
1,25D 20% insufficiency 26% Untreated Hormone 44% 36%
Nutritional Nutritional Vitamin D Vitamin D 36% Efficacy 38% Concerns
RayaldyTM Capsules expected to take significant market share in the CKD
BioTrends Research Group, Inc. December 2010 11
11
|
|
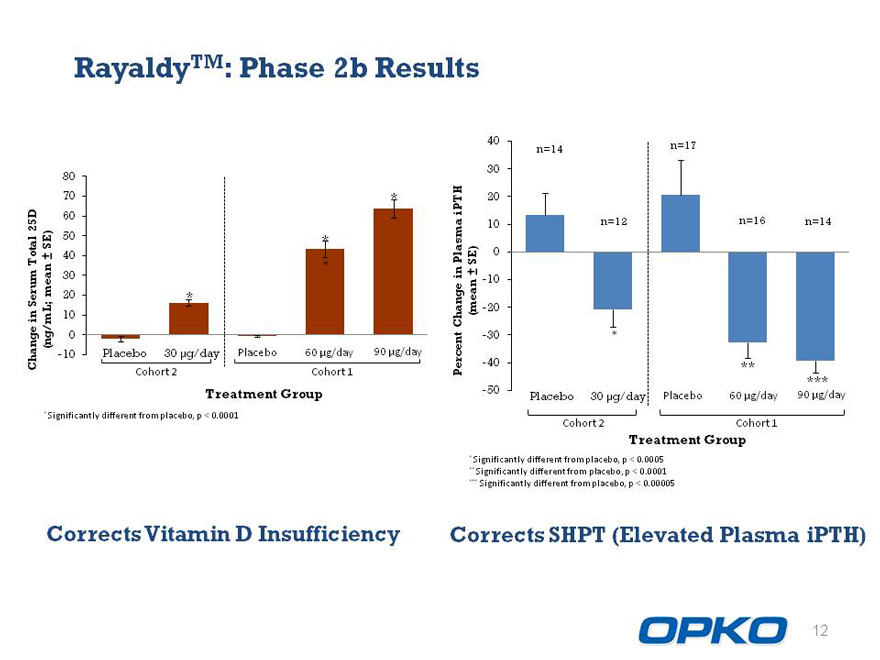
RayaldyTM: Phase 2b Results
40 n=14 n=17
30 80
H
70 20
60 n=16
25D 10 n=12 n=14
SE) 50
40 0
Total±
| * |
|
m ean 30 -10 |
Serum * in (mea Change
(ng/mL; *
Change Placebo 30 µg/day Placebo 60 µg/day 90 µg/day Cohort 2 Cohort 1 Percent
Treatment Group -50 90 µg/day
Placebo 30 µg/day Placebo 60 µg/day
| * |
|
Significantly different from placebo, p < 0.0001 |
Cohort 2 Cohort 1
Treatment Group
| * |
|
Significantly different from placebo, p < 0.0005 |
** Significantly different from placebo, p < 0.0001 *** Significantly different from placebo, p < 0.00005
Corrects Vitamin D Insufficiency
Corrects SHPT (Elevated Plasma iPTH)
12
|
|
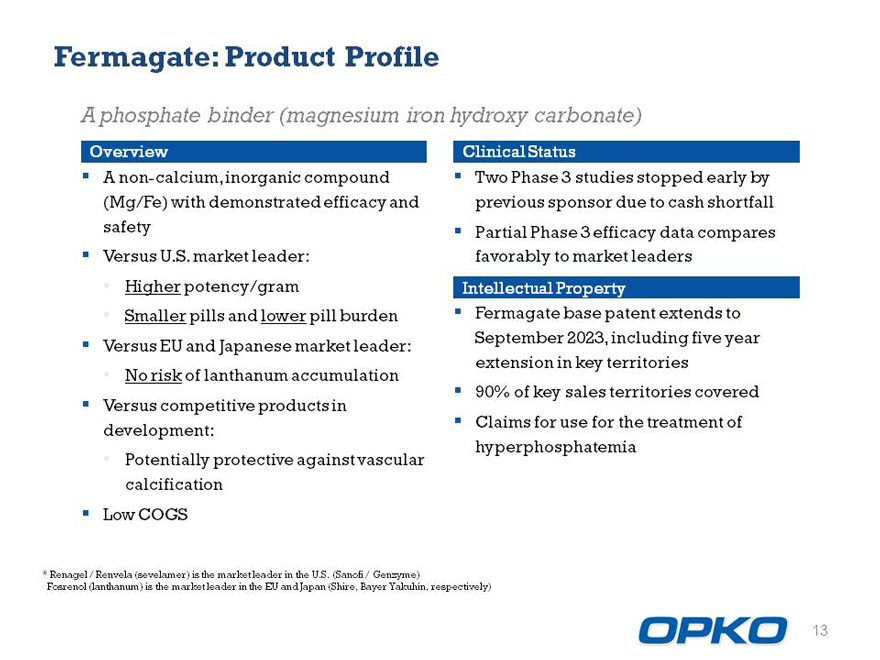
Fermagate: Product Profile
A phosphate binder (magnesium iron hydroxy carbonate)
Overview
A non-calcium, inorganic compound (Mg/Fe) with demonstrated efficacy and safety
Versus U.S. market leader:
Higher potency/gram
Smaller pills and lower pill burden
Versus EU and Japanese market leader:
No risk of lanthanum accumulation
Versus competitive products in development:
Potentially protective against vascular calcification
Low COGS
Clinical Status
Two Phase 3 studies stopped early by previous sponsor due to cash shortfall
Partial Phase 3 efficacy data compares favorably to market leaders Intellectual Property
Fermagate base patent extends to September 2023, including five year extension in key territories
90% of key sales territories covered
Claims for use for the treatment of hyperphosphatemia
| * |
|
Renagel / Renvela (sevelamer) is the market leader in the U.S. (Sanofi / Genzyme) |
Fosrenol (lanthanum) is the market leader in the EU and Japan (Shire, Bayer Yakuhin, respectively)
13
|
|
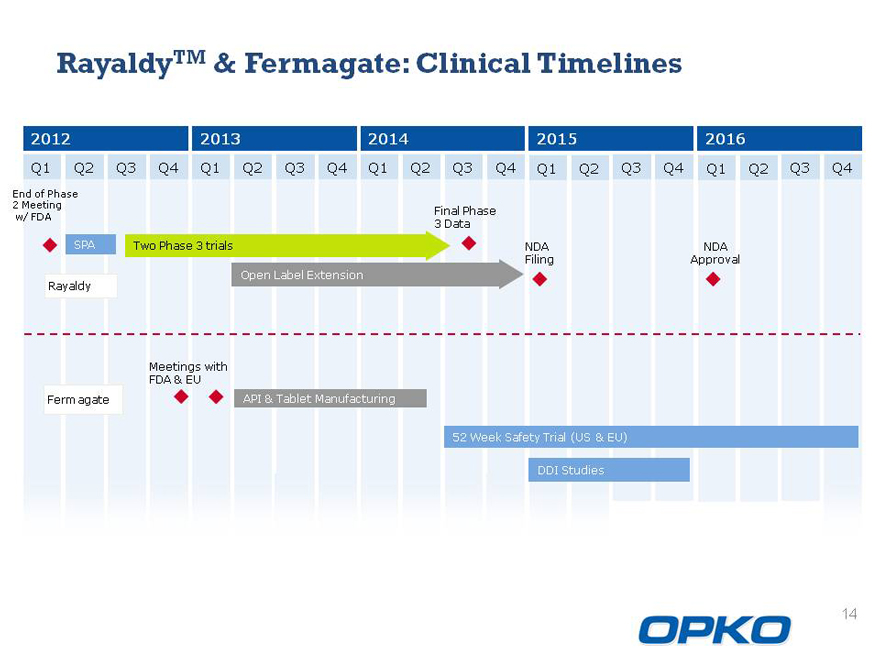
RayaldyTM & Fermagate: Clinical Timelines
2012 2013 2014 2015 2016
Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4
End of Phase
| 2 |
|
Meeting Final Phase w/ FDA |
| 3 |
|
Data |
SPA Two Phase 3 trials NDA NDA Filing Approval Rayaldy Open Label Extension
Meetings with FDA & EU
Fermagate API & Tablet Manufacturing
52 Week Safety Trial (US & EU) DDI Studies
14
|
|
Rolapitant out-licensed to Tesaro in December 2010
Schering-Plough (S-P) divested to OPKO as FTC condition for S-P merger with Merck
Payments of up to $121 million
Double-digit tiered royalties
Potentially best-in-class cancer supportive care product
Potent neurokinin-1(NK-1) receptor antagonist for chemotherapy-induced nausea and vomiting (CINV)
Single dose
Rapid onset
Long acting
Phase 3 program initiated following positive Phase 2 results
Proof of concept and dose identified in Phase 2 trial (n=454)
Five-day activity following single oral dose in patients treated with highly emetogenic chemotherapy (HEC)
Over 1,000 patients and healthy volunteers evaluated in Phase 1 and 2
Well accepted regulatory endpoints and clinical trial designs
Three global Phase 3 studies ongoing; 2 HEC (n=530) and 1 MEC (n=1350) with results expected during second half of 2013
15
|
|
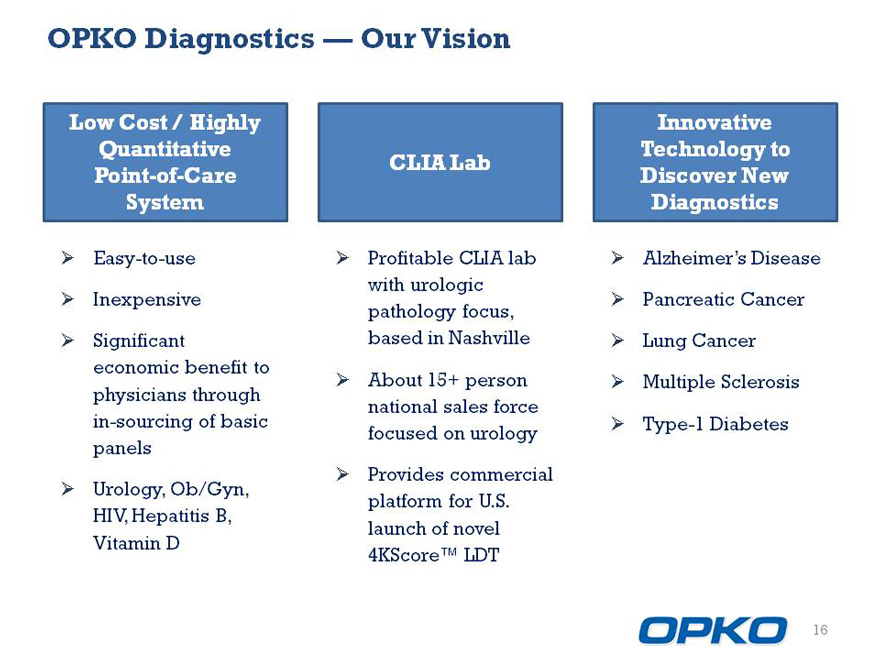
OPKO Diagnostics Our Vision
Low Cost / Highly Quantitative Point-of-Care
3/4 Easy-to-use
3/4 Inexpensive
3/4 Significant economic benefit to physicians through in-sourcing of basic panels
3/4 Urology, Ob/Gyn, HIV, Hepatitis B, Vitamin D
CLIA Lab
3/4 Profitable CLIA lab with urologic pathology focus, based in Nashville
3/4 About 15+ person national sales force focused on urology
3/4 Provides commercial platform for U.S. launch of novel 4KScore LDT
Innovative Technology to Discover New
3/4 Alzheimers Disease
3/4 Pancreatic Cancer
3/4 Lung Cancer
3/4 Multiple Sclerosis
3/4 Type-1 Diabetes
16
|
|
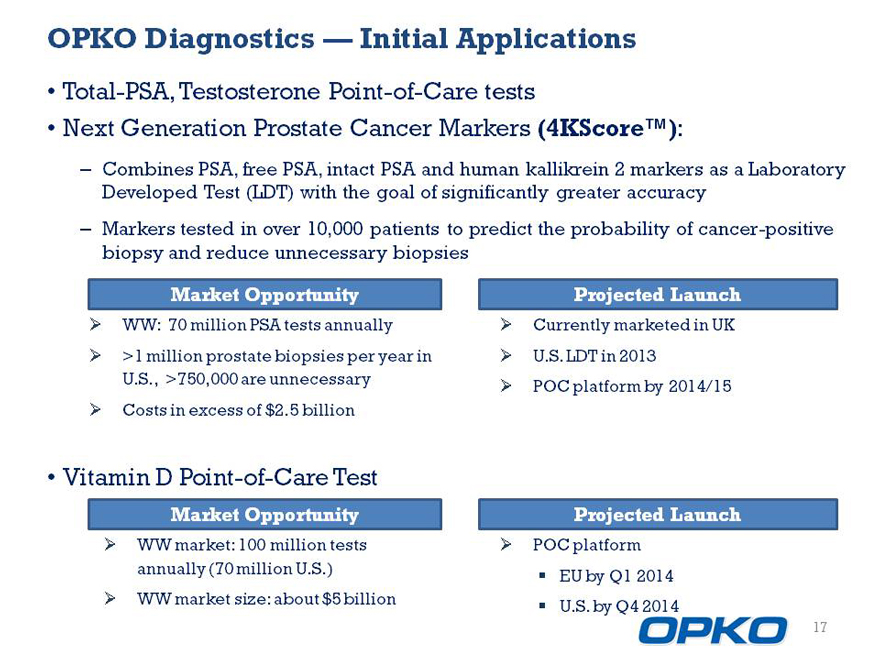
OPKO Diagnostics Initial Applications
Total-PSA, Testosterone Point-of-Care tests
Next Generation Prostate Cancer Markers :
Combines PSA, free PSA, intact PSA and human kallikrein 2 markers as a Laboratory
Developed Test (LDT) with the goal of significantly greater accuracy
Markers tested in over 10,000 patients to predict the probability of cancer-positive
biopsy and reduce unnecessary biopsies
Market Opportunity
3/4 WW: 70 million PSA tests annually
3/4 >1 million prostate biopsies per year in U.S., >750,000 are unnecessary
3/4 Costs in excess of $2.5 billion
Vitamin D Point-of-Care Test
3/4 WW market: 100 million tests annually (70 million U.S.)
3/4 WW market size: about $5 billion
3/4 Currently marketed in
3/4 U.S. LDT in 2013
3/4 POC platform by 2014/15
3/4 POC platform
EU by Q1 2014
U.S. by Q4 2014
17
|
|
Projected Launch
Key Requirements High Quantitative Performance / Low Cost
Injection-molded microfluidics
On-board delivery of multiple reagents
Multiplex platform
Sensitive proprietary amplification chemistry for microfluidics
Reliable standard optics
No sample prep
User-friendly sample introduction
Biohazard containment
18
|
|
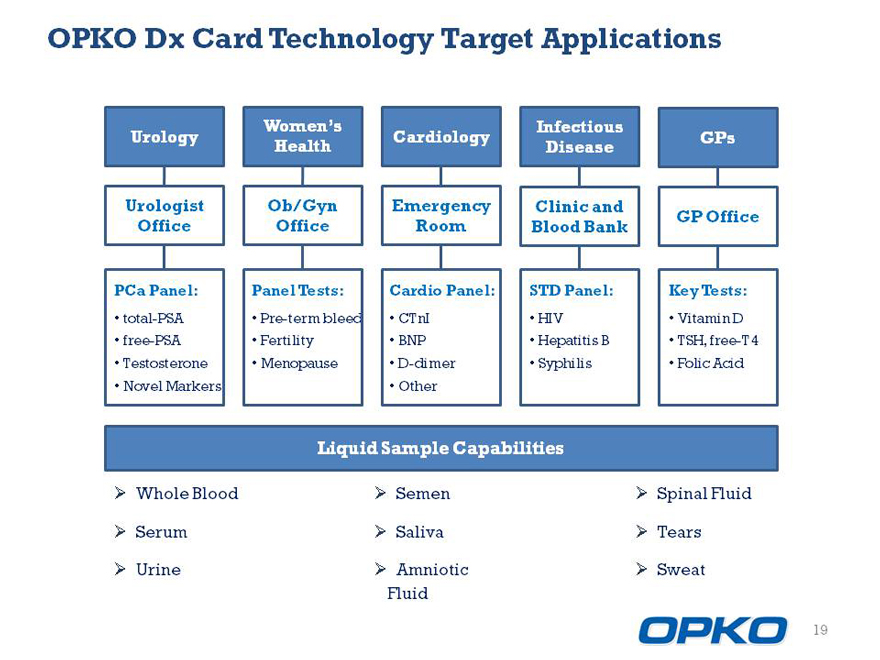
OPKO Dx Card Technology Target Applications
Womens Infectious
Urology Cardiology GPs Health Disease
Urologist Ob/Gyn Emergency Clinic and GP Office Office Office Room Blood Bank
PCa Panel: Panel Tests: Cardio Panel: STD Panel: Key Tests:
Liquid Sample Capabilities
3/4 Whole Blood 3/4 Semen 3/4 Spinal Fluid
3/4 Serum 3/4 Saliva 3/4 Tears
3/4 Urine 3/4 Amniotic 3/4 Sweat Fluid
19
|
|
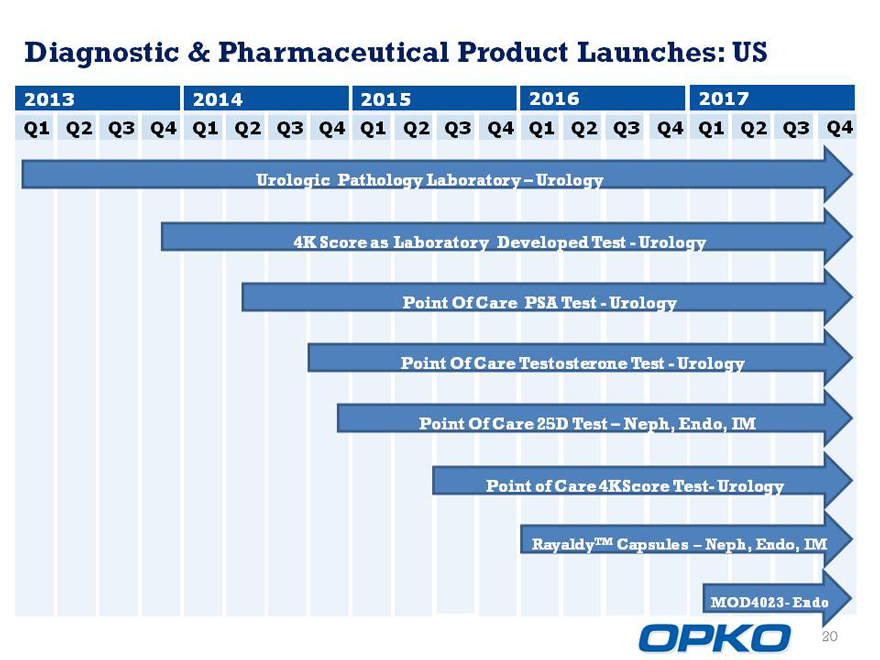
Diagnostic & Pharmaceutical Product Launches: US
2013 2014 2015 2016 2017
Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4
Urologic Pathology Laboratory Urology
4K Score as Laboratory Developed TestUrology
Point Of Care PSA TestUrology
Point Of Care Testosterone TestUrology
Point Of Care 25D Test Neph, Endo, IM
Point of Care 4KScore Test- Urology
RayaldyTM Capsules Neph, Endo, IM
MOD4023- Endo
20
|
|
OPKO European Union
Farmadiet Group
Established 20-year presence within EU
Based in Barcelona, Spain
Fully integrated specialty pharmaceutical entity
42-person sales force
Production/research facility in Banyoles, Spain
Broad pharmaceutical, nutritional and veterinary products lineup
Pharmaceuticals: 45 Products
Nutritionals: 70 Products
Veterinary : 50 Products
Access to promising products in development:
Citicoline (supports memory function)
25-Hydroxyvitamin D for animal use
21
|
|
OPKO Emerging Markets
Rapidly growing sales from >100 products
Acquired ALS Distribution Limited, the exclusive product distributor of Arama Laboratories, in April 2012
25 products across a range of therapeutic indications
Primarily branded ophthalmics, with expanding proprietary focus
Acquired February 2013
ANVISA licensed pharmaceutical company in most important growth market in South America
22
|
|
OPKO Emerging Markets (contd)
OPKO Israel
Acquired in December 2011
Develops and produces high value, high potency, difficult to make active pharmaceutical ingredients
FDA registered state of the art facility in Nesher, Israel
Acquired controlling interest in October 2012
Sci-B-Vac is a third-generation Hepatitis B vaccine encompassing:
Immunogenic regions of the three surface proteins of HBV
Faster onset of action (earlier seroconversion and seroprotection after the first and second doses)
98% protection in vaccinated patients following three injections at the recommended dose
Higher levels of anti-HBV antibodies
Sci-B-Vac is highly immunogenic and effective at low doses
Sci-B-Vac is currently marketed in nine countries
23
|
|
Strategic Investments
Proprietary Technologies with Significant Upside Potential
Discovery, Inc. (~16% equity interest*)
New approach to develop broad spectrum anti-viral drugs
Development program with Teva for Hepatitis C drug
. (~21% equity interest*)
RNA interference platform that down-regulates abnormal gene expression
RXI-109 in development to reduce or inhibit scar formation in the skin following
surgery
Therapeutics (~20% equity interest*)
New technology to produce human monoclonal antibodies libraries that are more complete
, LLC (~13% equity interest*)
Next gen technology to identify therapeutic antibody targets
(~10% equity interest)
Manufactures and sells branded pharmaceutical products, primarily in Russia and Baltic countries
Develop and commercialize OPKO products in Eastern Europe
, Inc. (~1% equity interest*)
Oncology-focused biopharmaceutical company founded by former executives of MGI Pharma
, Inc. (~ 4% equity interest*)
Developing innovative vascular devices
, Inc. (~1% equity interest*)
Dietary supplement BlueScience and antioxidant pterostilbene pTeroPure
OPKO distribution rights in Latin America
24
| * |
|
As of March 31, 2013 |
|
|
Additional Information About the PROLOR Biotech, Inc. (PROLOR) Transaction and Where to Find It
and does not constitute an offer of any securities for sale or a solicitation of an offer to buy any securities. In connection with the proposed transaction, OPKO will file with the SEC a registration statement on Form S-4 that will include a joint proxy statement of OPKO and PROLOR and that will also constitute a prospectus of OPKO. The definitive joint proxy statement/prospectus will be mailed to stockholders of OPKO and PROLOR. STOCKHOLDERS OF OPKO AND PROLOR ARE URGED TO READ THE JOINT PROXY STATEMENT/PROSPECTUS WHEN THEY BECOME AVAILABLE AND ANY OTHER RELEVANT DOCUMENTS FILED WITH THE SEC, AS WELL AS ANY AMENDMENTS OR SUPPLEMENTS TO THOSE DOCUMENTS, BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION. Investors and security holders will be able to obtain free copies of the registration statement and the definitive joint proxy statement/prospectus (when available) and other documents filed with the SEC by OPKO and PROLOR through the website maintained by the SEC at www.sec.gov. Free copies of the registration statement and the definitive joint proxy statement/prospectus (when available) and other documents filed with the SEC can also be obtained by directing a request to OPKO, attn: Steven D. Rubin, Executive Vice President Administration or Juan F. Rodriguez, Chief Financial Officer, at 305-575-4100, or PROLOR, attn; Shachar Shlosberger, PROLOR Biotech, Inc., 7 Golda Meir Street, Weizmann Science Park, Nes-Ziona, Israel 74140, at 866-644-7811.
This communication shall not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended.
OPKO, PROLOR and their respective directors and executive officers and other persons may be deemed to be participants in the solicitation of proxies in respect of the proposed transaction. Information regarding OPKOs directors and executive officers is available in its Form 10-K/A, which was filed with the SEC on April 29, 2013. Information regarding PROLORs directors and executive officers is available in its proxy statement for its 2013 annual meeting of stockholders, which was filed with the SEC on April 25, 2013. Other information regarding the participants in the proxy solicitation and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the definitive Joint Proxy Statement/Prospectus and other relevant materials to be filed with the SEC when they become available.
25
|
|
Jefferies 2013 Global Healthcare Conference June 4, 2013