EX-99.1
Published on June 3, 2014

Jefferies 2014 Global Healthcare Conference
June 3, 2014
Exhibit 99.1 |

2 |
OPKO
Important Products Available or Coming to Market Near Term
3
Diagnostics
4Kscore
Test blood test for personalized risk of high-grade
prostate cancer
CLIA-certified urological specialty laboratory
Pharmaceuticals
Vitamin
D
therapeutics
for
SHPT
*
Platform technology to make peptides and proteins long acting to
treat growth hormone deficiency, hemophilia, obesity, etc.
Calcium-free, magnesium-based phosphate binder
Approved third generation hepatitis B vaccine
*Secondary Hyperparathyroidism
Claros
®
1
immunoassay
system
for
rapid,
lab
quality
in-office
testing
(PSA,
Testosterone,
Vitamin
D) |

OPKO
Diagnostics Addressing Large Dx Markets
4
4Kscore
Test
Initially targeting pre-prostate biopsy market
PSA market 60 million tests globally
Recently launched in US at $395
Highly significant clinical data on 1,012 patients presented at AUA
plenary session May 18, 2014
Launch in Europe: September 2014
Claros
®
1 Analyzer and Sangia
Microfluidic Test Card
In office finger-stick blood analysis
Initial target assays in US:
PSA: 30 million tests, $750 M
Testosterone: 15 million tests, $525 M
Vitamin D: 70 million tests, $3.5 B |

5
DELIVERING BETTER
HEALTHCARE
CONVENIENT
LAB QUALITY
ACCURACY |
Finger stick blood
sample
Convenience
1-2
mins
10
mins
6 |
| Claros
1 Update
Testosterone
FDA: Pre-submission comments received from FDA
On track to file 510(k) in 2014
CE Mark: 4Q2014
PSA
FDA: Pre-submission response expected in August
Timing of 510k submission based on longitudinal trial requirements
CE Mark Update (Formulation and Chemistry): 4Q2014
Vitamin D
On track to support launch of Rayaldee 1Q2016
7 |
Challenges in Prostate Cancer Screening
High false positive rate of PSA
Patient Anxiety
1M Prostate biopsies in US
75% Negative or Low-Grade
Pain, bleeding, infection,
hospitalization
Recommendations to stop PSA
screening
8 |

4Kscore Test
Avoiding Unnecessary Prostate
Biopsies
The only
test to identify men with high-grade prostate
cancer from a blood sample
Combines results of multiple biomarkers to create a
patients personal risk score
Based on 10 years of clinical research by scientists at
Memorial Sloan-Kettering Cancer Center and leading
European cancer centers
Tested in over 10,000 men in 9 separate clinical studies
demonstrating a 27% -
82% biopsy reduction
Validated by OPKO in a prospective, blinded study of
1,012 men
9 |
4Kscore Test US Clinical Study
10
1,012 Patients Enrolled
Prospective Clinical Trial |
11
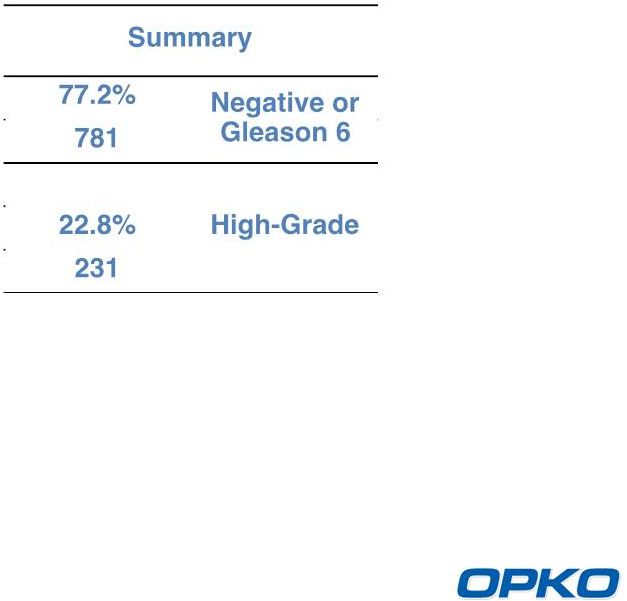
4Kscore Test Clinical Study Results in 1,012 Men
Discrimination: AUC = 0.82
Risk Calibration
Decision Curve Analysis
Biopsy reduction of 30% to 58% |
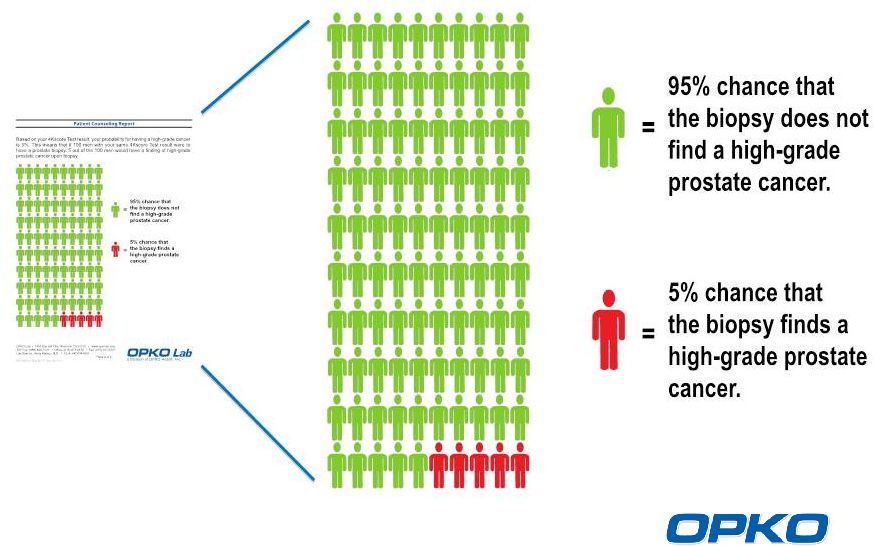
The
4Kscore Patient Counseling Report for a Result of 5%
12 |

The
4Kscore Test Conclusions Validated test based on a decade of clinical
research and a prospective, multi-institutional,
contemporary US clinical trial
Convenient blood test, cost $395
Excellent discrimination for high-grade cancer
(AUC = 0.82) and high net benefit for clinical use
Reduces 30
58% of biopsies
Provides a calibrated score for informed, shared
decision-making between urologist and patient
13 |
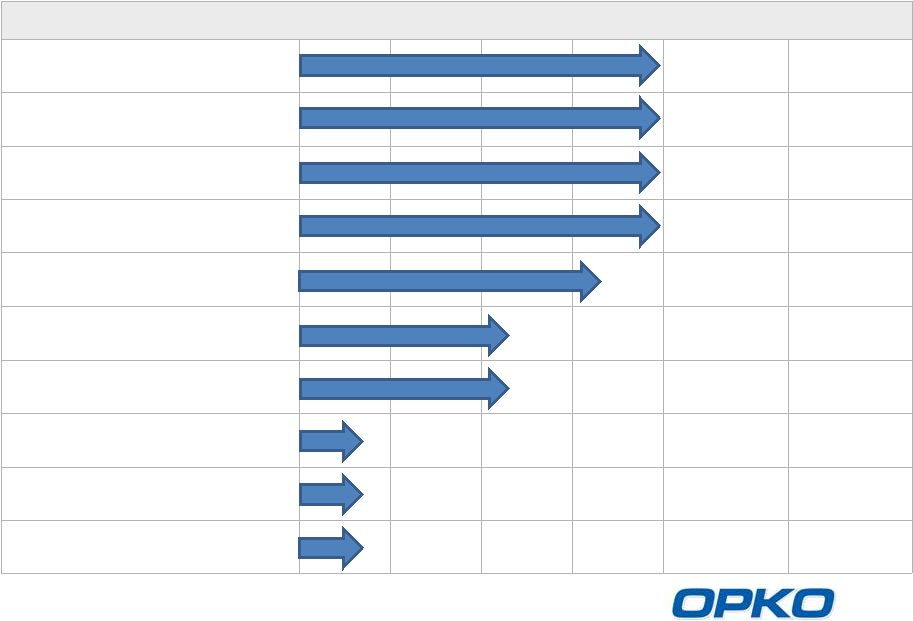
OPKO
Pharmaceuticals Advanced, Deep Pipeline
Product
Indication
Preclinical
Phase 1
Phase 2
Phase 3
Milestone
Market Size
Rayaldee
(CTAP101)
SHPT
(CKD Stage 3-4 Patients)
Phase 3 results
expected mid-
2014
$12.0 BN
hGH-CTP
hGH deficiency
$3.5 BN
Alpharen
(Fermagate)
Hyperphosphatemia
(CKD Stage 5 Patients)
$1.2 BN
Rolapitant
CINV
NDA submission
targeted mid-2014
$1.5 BN
Sci-B-Vac
Hepatitis B
(CKD Stage 5 Patients)
$0.2BN
Lunacalcipol
(CTA018)
Moderate to severe SHPT
(CKD Stage 5 Patients) &
Psoriasis
$1.5 BN
CTAP201
Mild to moderate SHPT
(CKD Stage 5 Patients)
$1.1 BN
Factor VIIa-CTP
Hemophilia
$1.7 BN
AntagoNAT
Platform
Cancer, CV, metabolic
and orphan disease
$1.0 BN
Oxyntomodulin
Diabetes, Obesity
$15 BN
Outlicensed to TESARO
14 |
15
Rayaldee (CTAP101)
A Late-Stage Investigational Drug
Product Overview
Oral formulation of 25D3*
addresses significant unmet need
Safe and effective treatment for
elevated PTH (SHPT) associated
with low 25D levels in Stage 3-4
CKD
Achieves reliable increases in
serum 25D and reductions in
plasma PTH
Lower risk of side effects
compared to active 1,25D
**
products
Potential for additional
indications including elderly,
osteoporosis & cancer
Clinical Status
Intellectual Property
* 25-Hydroxyvitamin D
** 1,25-Dihydroxyvitamin D
Clinical development guided by
prominent Scientific Advisory
Board
Top line phase 3 data available
in mid-2014
NDA filing in 1Q 2015
Rayaldee US patents issued,
protected through 2028
Additional global patents
allowed or pending
3 |
16
Rayaldee -
Commercial Opportunity
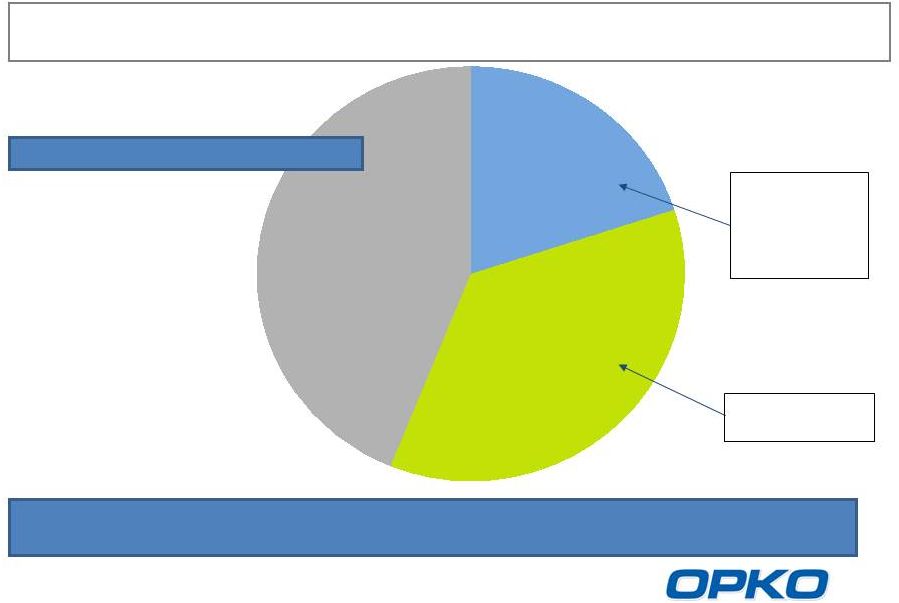
Source: BioTrends Research Group, Inc. December 2010
Untreated
26-44%
Vitamin D
Hormone
20-36%
Nutritional
Vitamin D
36-38%
Untreated
26-44%
Safety
concerns;
exacerbates
vitamin D
insufficiency
Efficacy
Concerns
Stage 3 & 4 CKD Treatment
Low
serum
25D
and
elevated
plasma
PTH
are
prevalent
in
CKD
Stage
3-4
patients
8 million CKD Stage 3-4 patients in the US
4 million patients with low serum 25D and high plasma PTH
Rayaldee is expected to take significant market share in Stage 3 and 4 CKD
patients suffering from SHPT a potential $12 billion revenue
opportunity
|
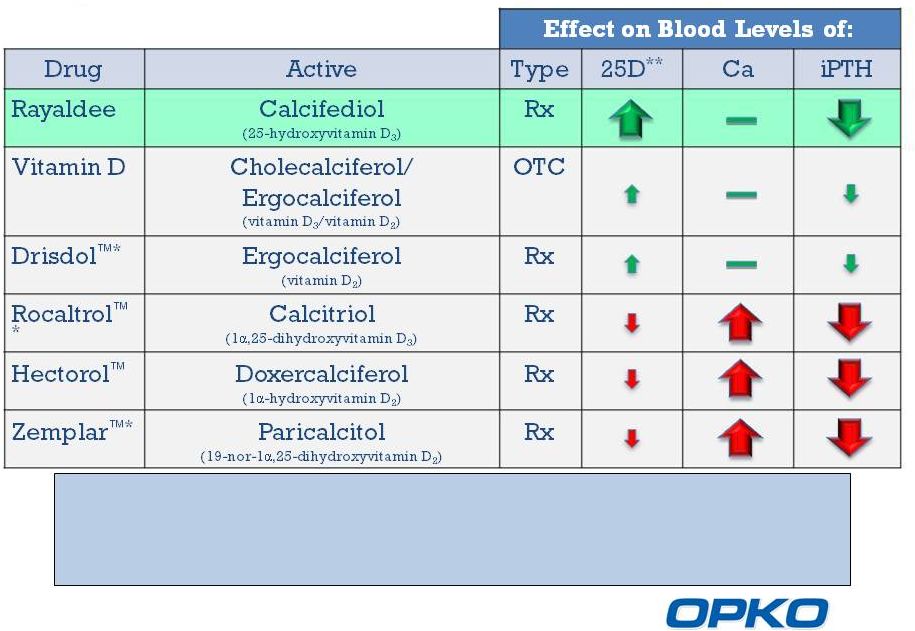
Rayaldee is expected to raise serum total 25-hydroxyvitamin D (25D) and
lower
plasma
iPTH
more
effectively
than
any
currently
marketed
over-the-
counter (OTC) or prescription (Rx) product without the risk of
hypercalcemia.
Comparison of Vitamin D Therapies for Stage 3-4 CKD
*And generics
**25-hydroxyvitamin D
17 |

CTP increases protein circulation time
Mercks long acting FSH-CTP (Elonva
):
o
Received EU marketing authorization in 2010; NDA filed Q3 2013
o
Single FSH-CTP injection replaces 7 daily FSH injections in fertility
treatment
Two licensees of CTP technology for human therapeutics:
o
Merck (holds license for 4 fertility-focused proteins )
o
OPKOs Biologics (holds license for all other rights)
18
CTP Technology: Clinically Validated Proprietary Platform
CTP
a natural sequence
created during evolution
to enhance the longevity
of peptides and proteins
without increasing toxicity
Any Short Acting
Protein
CTP
Long Acting
Protein |
|
$3.5 billion market, growing 5% annually
Once-a-week injection (current products require daily injections)
Small needle size (31 gauge) due to low viscosity
Competitive long acting formulations have high viscosity
Superb clinical, safety and immunogenicity profile
Human growth hormone is used for:
Growth hormone deficient children
Growth hormone deficient adults
Short stature
Off label
Orphan drug designation in the US & EU for children & adults
19
hGH-CTP Opportunity |
| hGH-CTP Clinical Development
Adult Pivotal Phase 3 trial (ongoing)
189 patients
Primary
efficacy
endpoint:
reduction
in
truncal
fat
mass
after
6
months
vs.
placebo
Secondary efficacy endpoints include:
Reduction in total body fat
Increase in lean body mass
Single pivotal trial required by FDA for BLA submission in 2016
Pediatric GHD Phase 2 trial (advanced stage)
Enrollment completed March 2014
4 cohorts:
3 dose levels of once-weekly hGH-CTP
Commercially available standard daily rhGH treatment
Key outcome: height velocity
Positive clinical data to be presented at ENDO meeting June 21-24,
2014
Phase 3 to commence by 1H2015
20 |
| FVIIa CTP: Long Acting for Treating Hemophilic Patients
21
$1.7 billion market
Growing 7% annually
Only 25% of patients are treated
Current product (NovoSeven®) requires frequent IV doses
3-4 times a day during bleeding episodes
1-2 times a day for prophylactic treatment
Pharmacological studies in hemophilic mice and dogs FVIIa-
CTP demonstrated:
Potential for substantial improvement in the quality of life of patients via
subcutaneous administration
Reduce frequency of injection during on-demand therapy
Enable prophylactic treatment while reducing the frequency of injections to
2-3 times a week
Phase 2a study in hemophilic patients: initiated H2 2014
Orphan drug designation in the US |
| MOD-6031: Long Acting Oxyntomodulin for Obesity
22
>$15 billion market
Growing rapidly
Oxyntomodulin
Natures Appetite Control Mechanism
Natural appetite suppressor
Secreted by the digestive system following food intake and induces satiety in
the brain
Increases glucose tolerance
Short acting
requires 3 injections per day
MOD-6031 Long Acting Oxyntomodulin-
weekly injection
studies in mutant obese mice and diet induced obese mice
demonstrated:
Significantly inhibited food intake and reduced body weight by reducing fat
Reduced cholesterol levels
Improved glycemic control
Phase 1 study to be initiated 1H2015
MOD-6031 is expected to provide superior long-term therapy
for obesity and diabetes type II patients |

Rolapitant
Potential Near-term Revenue Driver
Rolapitant out-licensed to Tesaro in December 2010
Payments of up to $121 million
Double-digit tiered royalties
Differentiated cancer supportive care product with $1.5B US Market
Opportunity
Potent neurokinin-1(NK-1) receptor antagonist for
chemotherapy-induced nausea and vomiting (CINV)
Opportunity to differentiate on convenience, market access and safety
Single dose
Lack of CYP 3A4 drug-drug interactions
Long acting
Oral and
IV formulations allow full market access
NDA submission targeted for mid-2014
All three Phase 3 trials (MEC
*
and HEC
**
) achieved primary endpoint
Primary endpoint: complete response (no emesis and no use of rescue
medication)
Third
Phase
3
trial
(HEC)
also
achieved
all
secondary
endpoints,
including:
Complete response in acute (0-24 hrs) and overall (0-120 hrs) phase of
CINV
No significant nausea
23
*Moderately emetogenic chemotherapy **Highly emetogenic
chemotherapy |
| Strategic Investments
ARNO Therapeutics,
Inc. (OTC: ARNI) (~5% equity interest)
Anti-progestins for breast (phase 2) , endometrial and prostate
cancers
Zebra Biologics,
Inc. (~19% equity interest)
Combinatorial antibody libraries based on function in human cell
screens
OAO Pharmsynthez
(MICE: LIFE) (~17% equity interest)
Russian developer and marketer of new drugs
RXi Pharmaceuticals
Corporation (NASDAQ: RXII)
(~17% equity interest)
sRNA to prevent hypertrophic scars (phase 2)
Cocrystal Pharma,
Inc. (OTC: COCP) (~16% equity interest)
New anti-virals (Hepatitis C, flu, dengue fever)
Fabrus,
Inc.
(~12% equity interest*)
Antibodies against difficult targets (e.g., G protein-coupled receptor, ion
channels)
Neovasc,
Inc. (NASDAQ: NVCN) (~ 6% equity interest)
Cardiology devices
ChromaDex,
Inc.
(OTC: CDXC) (~2% equity interest)
New nutritional supplement APIs
24
Proprietary Technologies with Significant Upside Potential
(As of March 31, 2014)
*
Merger with Senesco Technologies, Inc. (OTC: SNTI) completed May 19, 2014
|