EX-99.4
Published on June 24, 2014
Exhibit 99.4
|
|
Exhibit 99.4
June 2014
1
|
|
Cautionary Statement
This presentation contains forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA), which statements
may be identified by words such as expects, plans, projects, will, may, anticipates, believes,
should, intends, estimates, and other words of similar meaning, in cluding statements regarding expected results and benefits of Lagova, including its safety and efficacy, whether clinical
trials for adult and pediatric growth hormone deficiency will generate data to support marketing approval, whether a single
injection of Lagova can replace seven consecutive daily injections of currently marketed hGH, whether Lagova will have low immunogenecity, the expected commencement date for the Phase 3 clinical trial for Lagova in pediatric patients, whether Lagova will be successfully developed or commercialized, expectations regarding Lagova and our other products in development and their market potential, whether Lagova has competitive advantages over other products, as well as other non-historical statements about our expectations, beliefs or intentions regarding our business, technologies and products, financial
condition, strategies or prospects. Many factors could cause our actual activities or results to differ materially from
the activities and results ant icipated in forward-looking statements. These factors include those described in our filings with the Securities and Exchange Commission, as well
as the risks inherent in funding, developing and obtaining regulatory approvals of new, commercially-viable and competitive products and treatments, including the risks that the Phase 3 clinical trials for the Lagova product may not be successful or achieve the expected results or effectiveness, and may not generate data that would
support the approval or marketing of this product for the indications being studied, that others may develop products which are
superior to Lagova, and that Lagova may not have advantages or prove to be superior over presently marketed products or products introduced in the future.
In addition, forward-looking statements may also be adversely affected by general market factors, competitive product development, product availability,
federal and state regulations and legislation, the regulatory process for new products and indications, manufacturing issues that may arise, patent positions and litigation, among other factors, including
all of the risks identified under the heading Risk Factors in OPKOs Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. The forward-looking statements contained in this presentation speak only as of the date the statements were made, and we do not undertake
any obligation to update forward-looking statements. We intend that all forward-looking statements be subject to the safe-harbor provisions of the PSLRA.
2
|
|
Ron G. Rosenfeld, MD
Professor of Pediatrics (emeritus), Stanford University
Professor of Pediatrics, Oregon Health & Science University (emeritus)
President, STAT5 Consulting, LLC
3
|
|
Lagova
The Long Acting Human Growth Hormone ENDO 2014
OPKO Biologics Ltd. Nes-Ziona, Israel
|
|
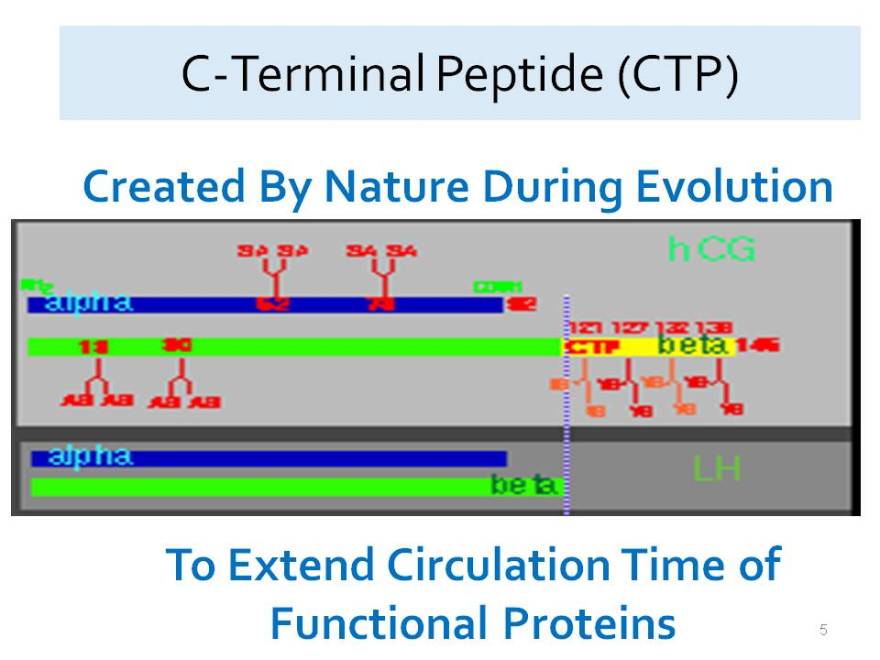
C-Terminal Peptide (CTP)
Created By Nature During Evolution
To Extend Circulation Time of Functional Proteins
5
|
|
CTP: Clinically Validated Technology
CTP technology has been used in an approved product (Elonva®, Once weekly FSH-CTP, Merck)
6
|
|
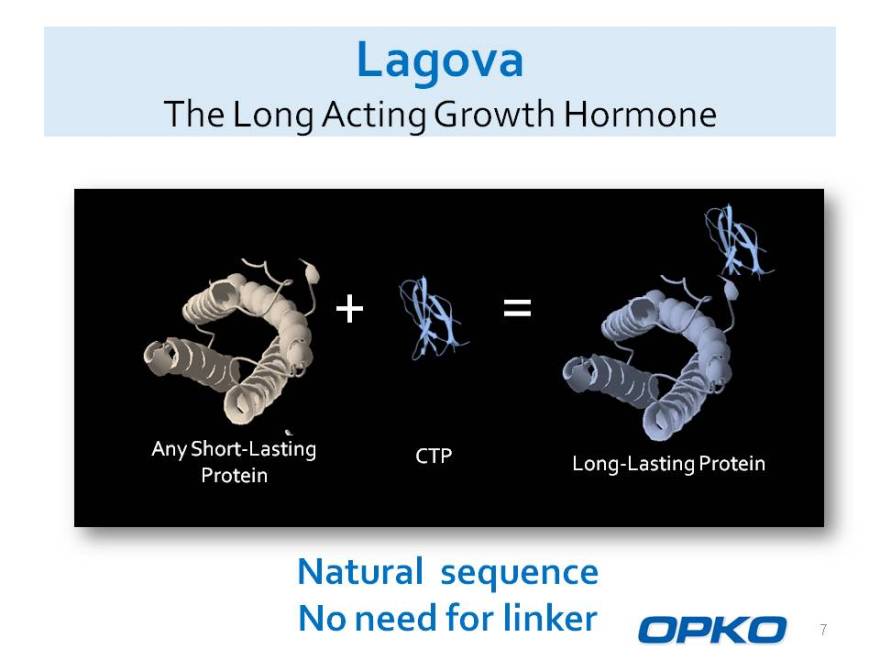
Lagova
The Long Acting Growth Hormone
+ =
Any Short-Lasting CTP
Long-Lasting Protein Protein
Natural sequence No need for linker
7
|
|
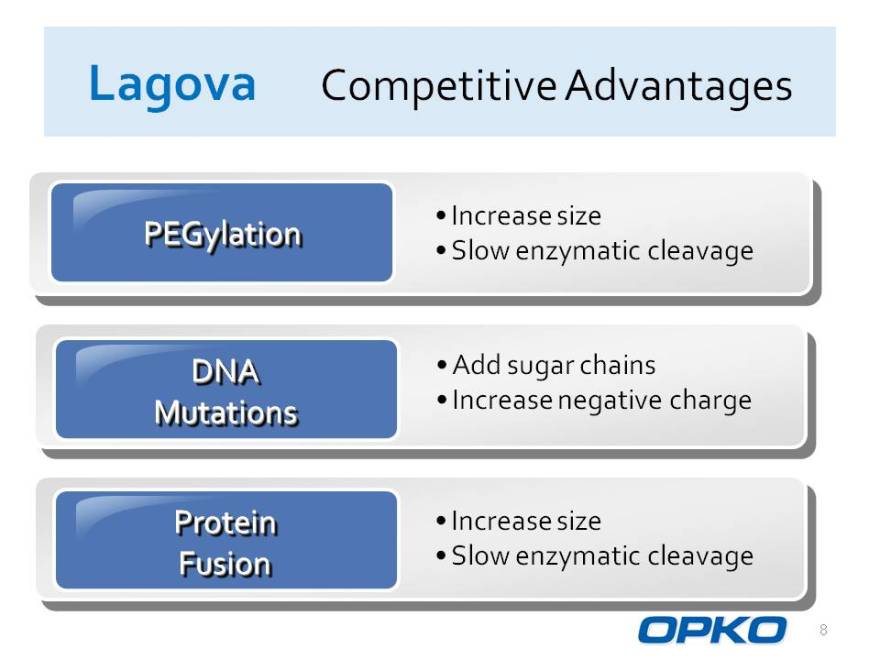
Lagova Competitive Advantages
Increase size
PEGylation
Slow enzymatic cleavage
DNA Add sugar chains Mutations Increase negative charge
Protein Increase size
Fusion Slow enzymatic cleavage
8
|
|
Lagova Competitive Advantages
Lagova Potentially non-immunogenic
Lagova ~75% native hGH
Lagova Ease of administration
9
|
|
Lagova The Long Acting hGH
Global multi-center Phase 3 study in growth hormone deficient adults is ongoing
10
|
|
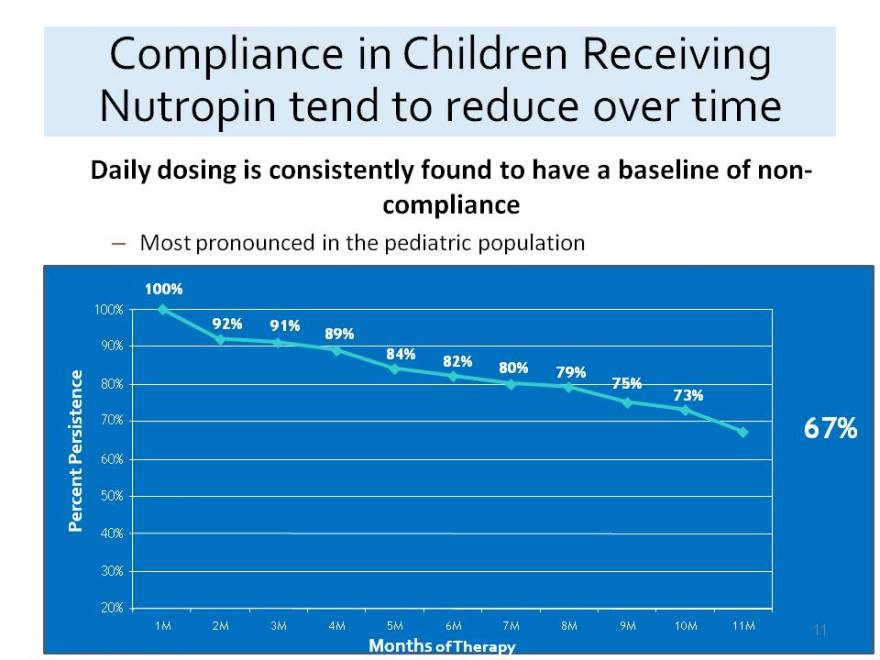
Compliance in Children Receiving Nutropin tend to reduce over time
Daily dosing is consistently found to have a baseline of non-compliance
Most pronounced in the pediatric population
100%
100%
92% 91% 89%
90%
84% 82% e 80% 79% 75%
80%
73%
Persistenc 70% 67%
60%
Percent 50%
40%
30%
20%
1M 2M 3M 4M 5M 6M 7M 8M 9M 10M 11M
Months of Therapy
11
|
|
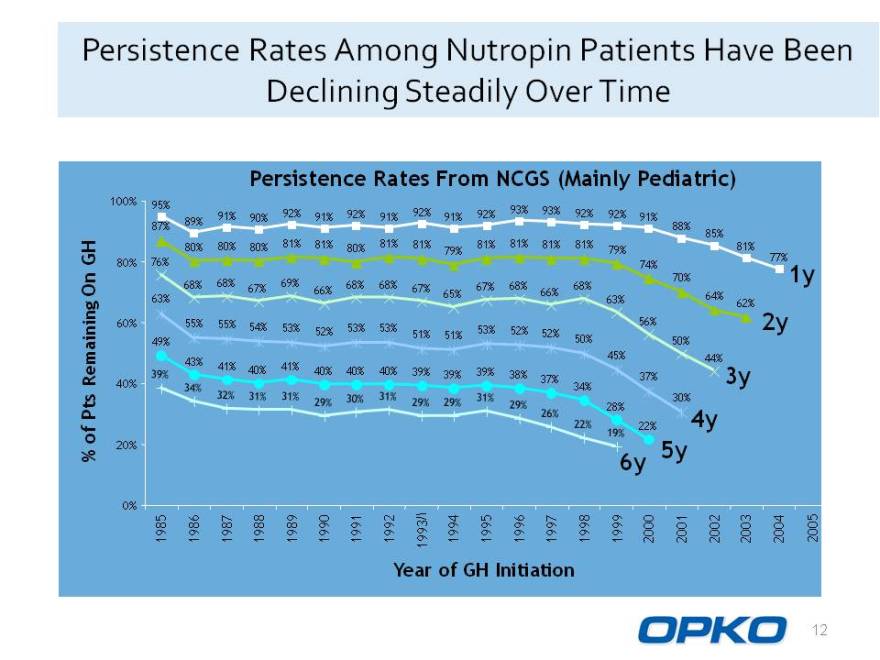
Persistence Rates Among Nutropin Patients Have Been Declining Steadily Over Time
Persistence Rates From NCGS (Mainly Pediatric)
100% 95%
92% 92% 92% 92% 93% 93% 92% 92% 91% 90% 91% 91% 91% 91% 87% 89% 88% 81% 81% 81% 85% 80% 80% 80% 81% 80% 81% 81% 81% 81% 79% 81% 79% GH 80% 76% 77% 74% n 70% 1y 68% 68% 67% 69% 68% 68% 67% 67% 68% 68% O 66% 65% 66% 63% 63% 64% 62% 60% 55% 55% 56% 2y 54% 53% 52% 53% 53% 53% 52% 51% 51% 52% 49% 50% 50% 45% 44% 43% 41% 41% emaining 40% 40% 40% 40% 39% 39% 39% 38% R 37% 37% 3y
40% 34% 39% 34% 32% 31% 31% 29% 30% 30% 30% 31% 29% 29% 31% 29% 26% 22% 19%
30%
Pts 28%
22% 4y
20% 5y
6y
0%
1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005
Year of GH Initiation
12
|
|
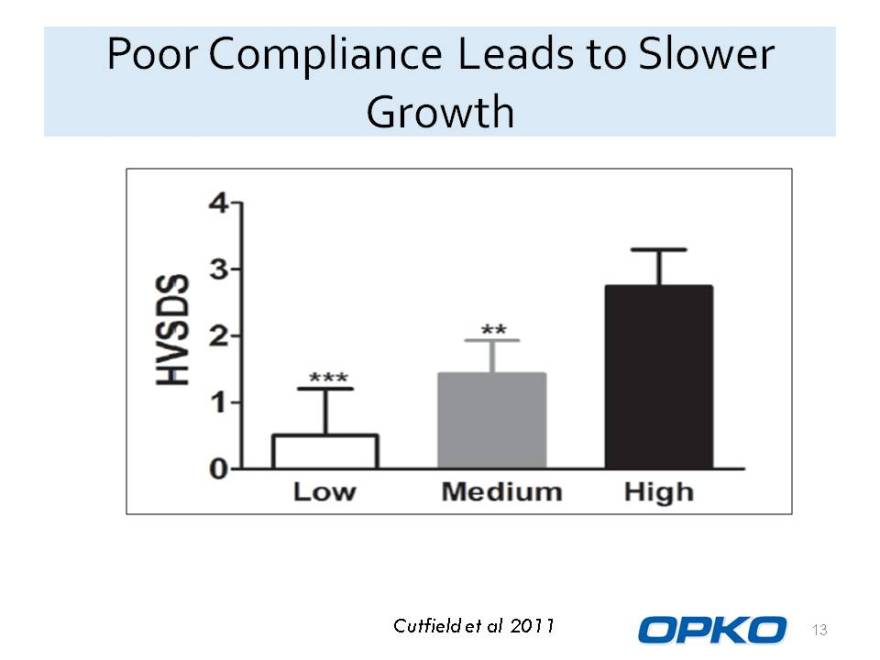
Poor Compliance Leads to Slower Growth
Cutfield et al 2011 HVSDS Low Medium High 0 1 2 3 4
13
|
|
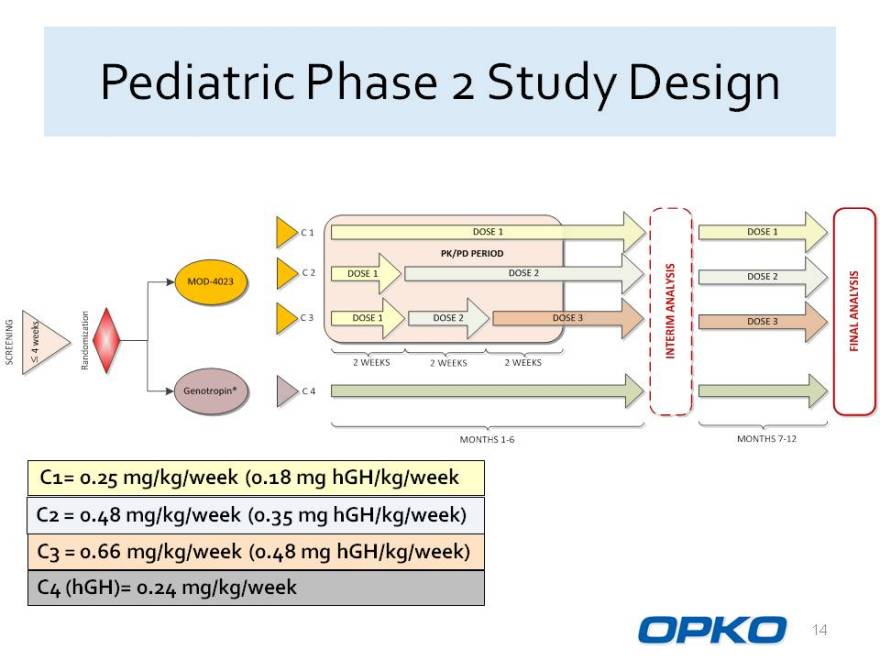
Pediatric Phase 2 Study Design
C1= 0.25 mg/kg/week (0.18 mg hGH/kg/week
C2 = 0.48 mg/kg/week (0.35 mg hGH/kg/week) C3 = 0.66 mg/kg/week (0.48 mg hGH/kg/week) C4 (hGH)= 0.24 mg/kg/week
SCREENING 4 weeks MOD-4023 Genotropin* C1 C2 C3 C4 2 WEEKS 2 WEEKS 2 WEEKS DOSE1 PK/PD PERIOD DOSE 1 DOSE 2 DOSE 1 DOSE 2 DOSE3 INTERIM ANALAYSIS DOSE 1 DOSE 2 DOSE 3 MONTHS 7-12 FINAL ANALYSIS MONTHS 7-12
14
|
|
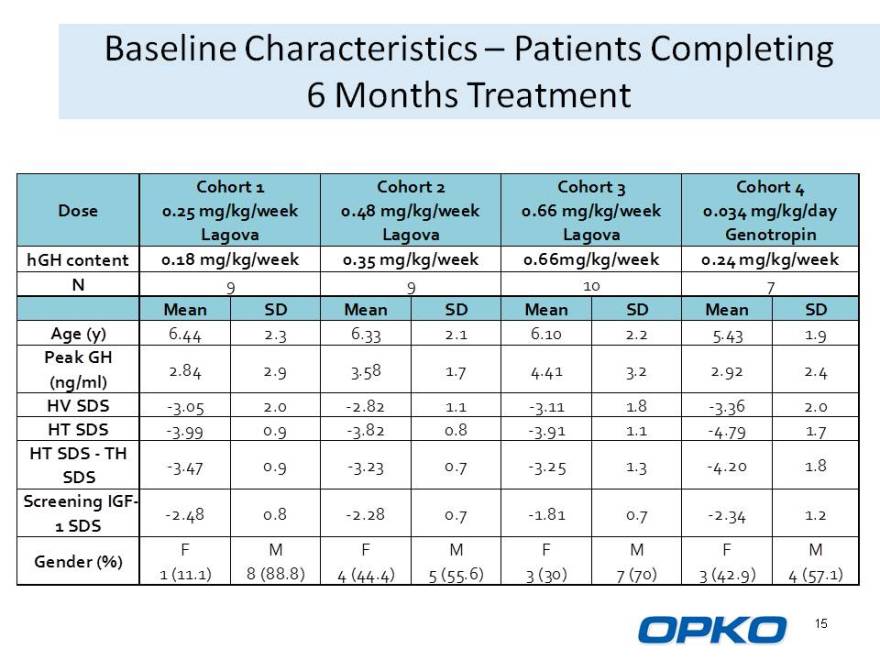
Baseline Characteristics Patients Completing
6 Months Treatment
Cohort 1 Cohort 2 Cohort 3 Cohort 4 Dose 0.25 mg/kg/week 0.48 mg/kg/week 0.66 mg/kg/week 0.034 mg/kg/day Lagova Lagova Lagova Genotropin hGH content 0.18 mg/kg/week 0.35 mg/kg/week 0.66mg/kg/week 0.24 mg/kg/week N 9 9 10 7
Mean SD Mean SD Mean SD Mean SD Age (y) 6.44 2.3 6.33 2.1 6.10 2.2 5.43 1.9
Peak GH
2.84 2.9 3.58 1.7 4.41 3.2 2.92 2.4
(ng/ml)
HV SDS -3.05 2.0 -2.82 1.1 -3.11 1.8 -3.36 2.0 HT SDS -3.99 0.9 -3.82 0.8 -3.91 1.1 -4.79 1.7
HT SDS - TH
-3.47 0.9 -3.23 0.7 -3.25 1.3 -4.20 1.8
SDS Screening IGF-
-2.48 0.8 -2.28 0.7 -1.81 0.7 -2.34 1.2
1 SDS
F M F M F M F M
Gender (%)
1 (11.1) 8 (88.8) 4 (44.4) 5 (55.6) 3 (30) 7 (70) 3 (42.9) 4 (57.1)
15
|
|
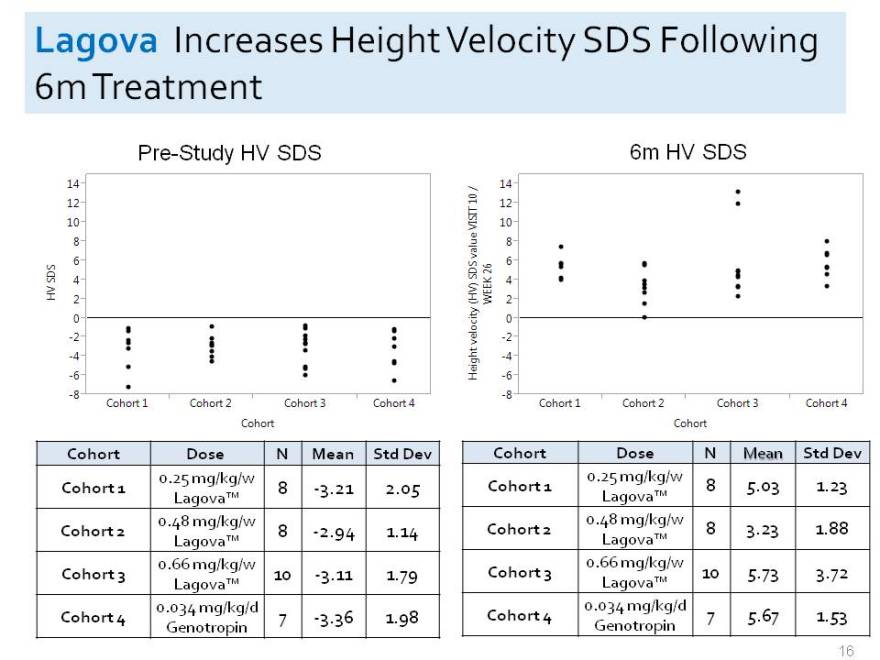
Lagova Increases Height Velocity SDS Following
6m Treatment
Pre-Study HV SDS 6m HV SDS
Cohort Dose N Mean Std Dev Cohort Dose N Mean Std Dev
0.25 mg/kg/w 0.25 mg/kg/w
Cohort 1 8 -3.21 2.05 Cohort 1 8 5.03 1.23
Lagova Lagova 0.48 mg/kg/w 0.48 mg/kg/w
Cohort 2 8 -2.94 1.14 Cohort 2 8 3.23 1.88
Lagova Lagova 0.66 mg/kg/w 0.66 mg/kg/w
Cohort 3 10 -3.11 1.79 Cohort 3 10 5.73 3.72
Lagova Lagova 0.034 mg/kg/d 0.034 mg/kg/d
Cohort 4 7 -3.36 1.98 16 Cohort 4 7 5.67 1.53
Genotropin Genotropin 6HV SDS Height velocity (HV) SDS value VISIT 10/ WEEK 26
14
12 10 8 6 4 2 0 -2 -4 -6 -8 Cohort 1 Cohort 2 Cohort 3 Cohort 4 Cohort
14
12 10 8 6 4 2 0 -2 -4 -6 -8 Cohort 1 Cohort 2 Cohort 3 Cohort 4 Cohort
|
|
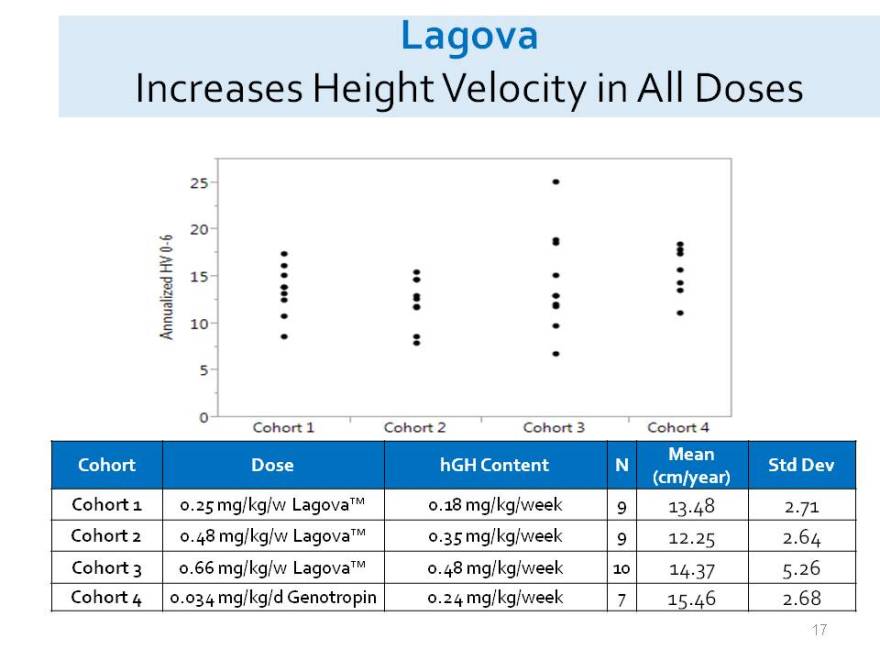
Lagova
Increases Height Velocity in All Doses
Mean
Cohort Dose hGH Content N Std Dev (cm/year) Cohort 1 0.25 mg/kg/w Lagova 0.18 mg/kg/week 9 13.48 2.71 Cohort 2 0.48 mg/kg/w Lagova 0.35 mg/kg/week 9 12.25 2.64 Cohort 3 0.66 mg/kg/w Lagova 0.48 mg/kg/week 10 14.37 5.26 Cohort 4 0.034 mg/kg/d Genotropin 0.24 mg/kg/week 7 15.46 2.68
25 20 15 10 5 0 Cohort 1 Cohort 2 Cohort 3 Cohort 4 Cohort
17
|
|
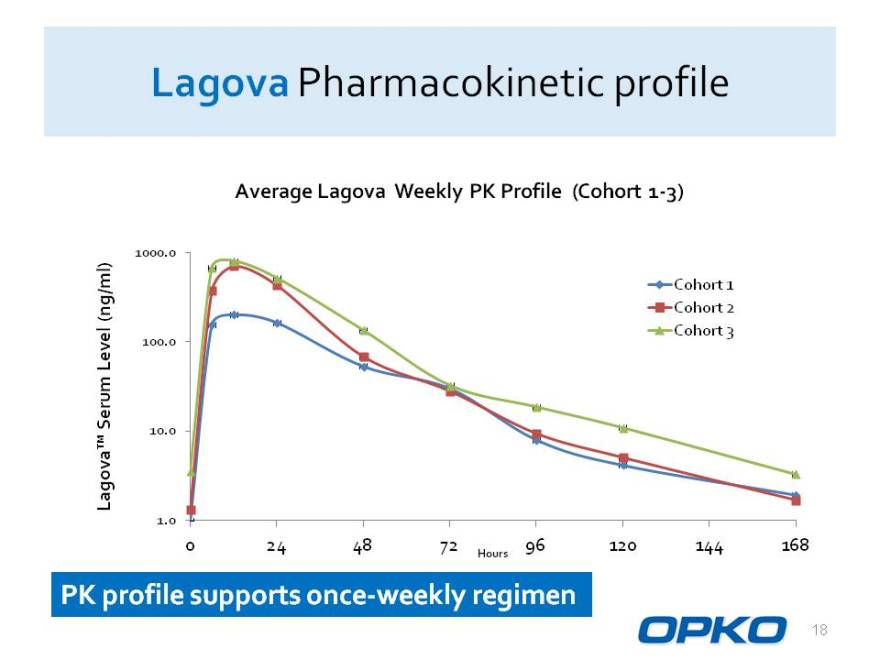
Lagova Pharmacokinetic profile
Average Lagova Weekly PK Profile (Cohort 1-3)
1000.0 ml) Cohort 1 Cohort 2
(ng/
100.0 Cohort 3
Level
Serum va 10.0
Lago
1.0
0 24 48 72 96 120 144 168
Hours
PK profile supports once-weekly regimen
18
|
|
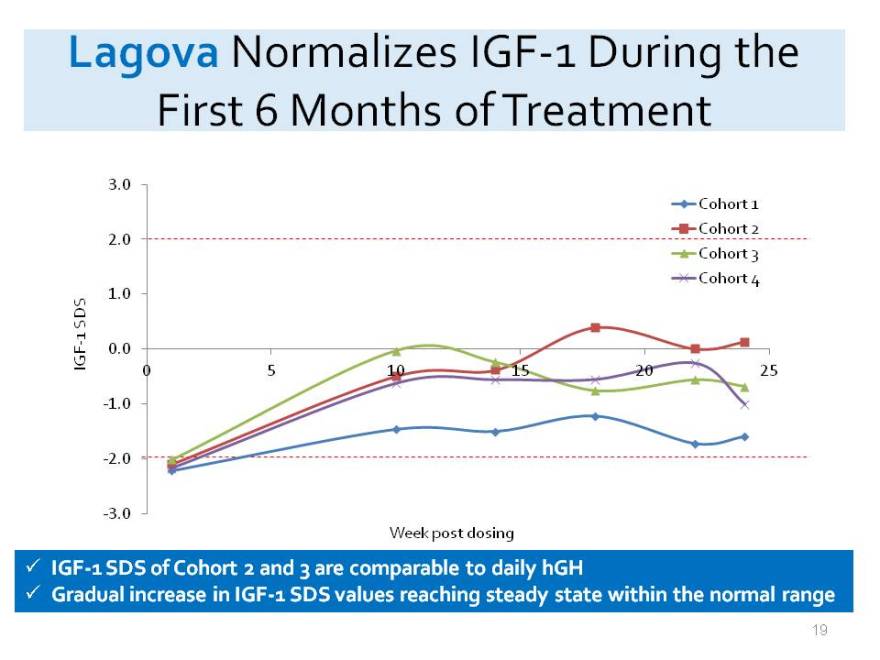
Lagova Normalizes IGF-1 During the First 6 Months of Treatment
3.0
Cohort 1 Cohort 2
2.0
Cohort 3 Cohort 4 SDS 1.0 1 IGF - 0.0
0 5 10 15 20 25 -1.0
-2.0
-3.0
Week post dosing
IGF-1 SDS of Cohort 2 and 3 are comparable to daily hGH
Gradual increase in IGF-1 SDS values reaching steady state within the normal range
19
|
|
Promising Safety Profile
No serious adverse events
No lipoatrophy
No clinically significant local tolerability issues were identified.
Comparable rate of AEs between Lagova groups and control group
20
|
|
Lagova The Long Acting hGH
Study supports safety and efficacy and allows selection of a dose for a Phase 3 study which is expected to start in 2015
21
|
|
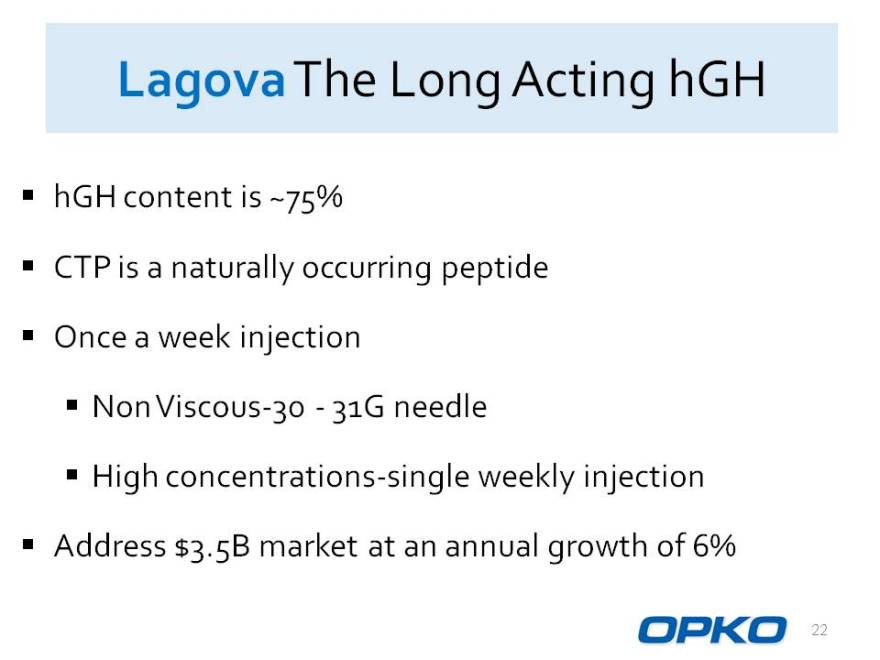
LagovaThe Long Acting hGH
hGH content is ~75%
CTP is a naturally occurring peptide
Once a week injection
Non Viscous-30 -31G needle
High concentrations-single weekly injection Address $3.5B market at an annual growth of 6%
22
|
|
Thank You
23