EX-99.1
Published on January 14, 2015

33
rd
Annual J.P. Morgan Healthcare Conference
January 2015
Exhibit 99.1 |
| 2
Cautionary Statement
This presentation contains "forward-looking statements, as that
term is defined under the Private Securities Litigation Reform Act of 1995
(PSLRA), which statements may be identified by words such as expects,
plans, projects, will, may, anticipates, believes,
should, intends, estimates,
"potential" and other words of similar meaning, including statements regarding our estimated revenues
and financial projections, our ability to achieve high levels of growth, the
potential for our products under development, the potential of the
4Kscore to reduce prostate biopsies by 30-58% and predict the risk of
aggressive prostate cancer, our ability to develop, test and launch new
products, the expected timing of the clinical studies and regulatory submissions relating to our products under development, the
outcome of our clinical trials and validation studies and that such outcomes
will support commercialization, the expected market penetration and size
of the market for our products under development, including without limitation, Rolapitant, Rayaldee (CTAP-101), hGH-CTP,
the 4Kscore, and our point-of-care diagnostic products for
Total-PSA, testosterone, and Vitamin D, the potential benefits of our products
under development, including whether the 4Kscore will improve selection of
candidates for prostate biopsy, predict the risk of distant
metastases, and result in $2 to 4 billion in healthcare savings, whether
MOD-6031 will provide superior long-term therapy for obesity and
Type II diabetes patients, our ability to successfully commercialize our product
candidates such as Rolapitant, the 4Kscore, Rayaldee (CTAP- 101) and
hGH-CTP, as well as products for other markets such as urology, womens health, cardiology, oncology, iPTH, and infectious
disease, whether we will be able to develop Rayaldee (CTAP-101) for
additional indications and whether Rayaldee (CTAP-101) will take
significant market share in Stage 3 and 4 CKD patients with SHPT, whether
Rayaldee (CTAP-101) will raise serum total 25-hydroxyvitamin D
(25D) more effectively than any over-the-counter (OTC) or prescription
(Rx) product currently marketed without the risk of hypercalcemia,
whether we can reach more than half of the CKD population with a small sales
force, expectations regarding patent coverage, the expected timing for
commencing, completing and obtaining results for our clinical trials, the timing for release of trial data and seeking and obtaining
FDA and European regulatory approvals, and the timing of commercial launch of
our product candidates, as well as other non-historical statements.
These forward-looking statements are only predictions and reflect our views as of the date they were made, and we undertake
no obligation to update such statements. Such statements are subject to many
risks and uncertainties that could cause our activities or actual results
to differ materially from the activities and results anticipated in forward-looking statements, including risks inherent in funding,
developing and obtaining regulatory approvals of new, commercially-viable
and competitive products and treatments, general market factors,
competitive product development, product availability, federal and state regulations and legislation, and integration issues arising
from the transactions, delays associated with development of novel technologies,
unexpected difficulties and delays in validating and testing product
candidates, the regulatory process for new products and indications, manufacturing issues that may arise, the cost of funding
lengthy research programs, the need for and availability of additional capital,
the possibility of infringing a third partys patents or other
intellectual property rights, the uncertainty of obtaining patents covering our
products and processes and in successfully enforcing them against third
parties, and the possibility of litigation, among other factors, including all of the risks identified under the heading Risk Factors
in our Annual Report on Form 10-K and other filings with the Securities and
Exchange Commission.
|
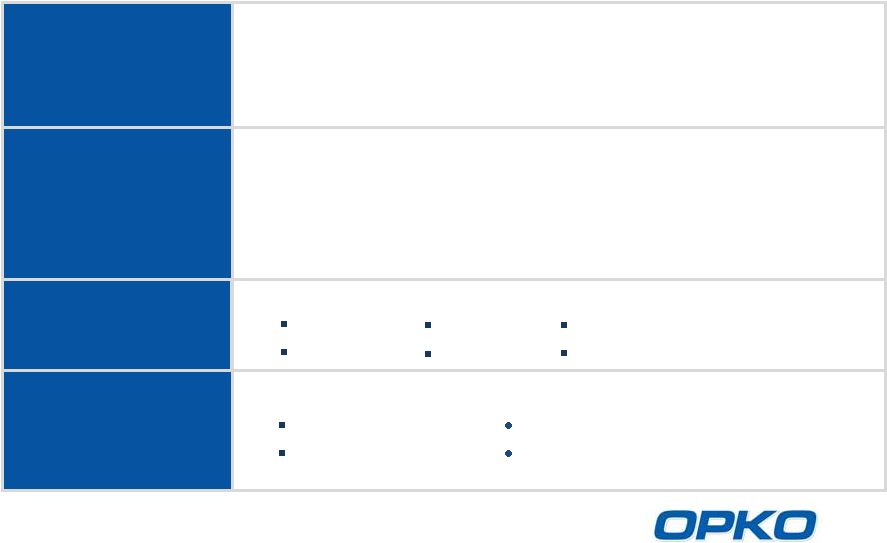
OPKO Health
Focus on Large Market Potential Products
Diagnostics
Claros
®
1 immunoassay system for rapid, lab quality in-office testing
4Kscore
blood test for aggressive prostate cancer risk
Pharmaceuticals
New Vitamin D products for SHPT
Platform technologies to make peptides and proteins long-acting
for growth hormone deficiency, hemophilia, obesity
Calcium-free, magnesium-based phosphate binder
Approved third generation hepatitis B vaccine
Opportunistic
Investments
Innovative technologies
Antibodies
RNAi
Anti-virals
Cardiovascular devices
International
Markets
Established businesses in:
Mexico
Chile
Spain
Brazil
Israel
Uruguay
3 |

4Kscore Test to Identify Risk for Aggressive
Prostate Cancer
Two types of prostate cancer:
Low grade, non-lethal
Aggressive, lethal
4Kscore: ONLY
blood test to predict risk of aggressive form
Backed by 10 years of clinical research at Memorial Sloan-
Kettering Cancer Center
Validated by OPKO in a prospective, blinded study of 1,012
men
Global market for pre-biopsy test (not screening): $800M
Use of 4Kscore would reduce 30-58% of unnecessary prostate
biopsies
Healthcare savings of $2-4 billion in the US
4 |
| 4Kscore Commercial Update
4Kscore US clinical trial manuscript in press (Eur. Urol.)
4Kscore CPT code application accepted by AMA CPT
Editorial Board (MAAA Category 3)
4Kscore was discussed at NCCN Guidelines meeting
Nov. 2014 for potential inclusion in 2015 guidelines
Over 465 US urologists have used the 4Kscore test in
routine practice
Launched by OPKO in Europe September 15, 2014;
samples tested at University of Barcelona
barnaclinic+
Mexico launch in January 2015; samples will be tested at
OPKO Lab in Nashville, TN
5 |
Claros
®
1: Rapid Testing in the Physician Office
10
mins
6
Finger stick blood
sample
1-2
mins |
| Claros
1 Platform Addresses Large Dx Markets
Testosterone
15 million US tests: $525 M
510(k) filing in 2Q2015
PSA
30 million US tests: $750 M
Intended use to focus on screening (early detection) claim
PMA filing in 1Q2016
Vitamin D
70 million US tests: $3.5 B
On track to support launch of Rayaldee 1Q2016
Lyme Disease
Claros 1 selected as platform for NIH funded development
OPKO has exclusive option to diagnostic test ($120M US Market)
7 |
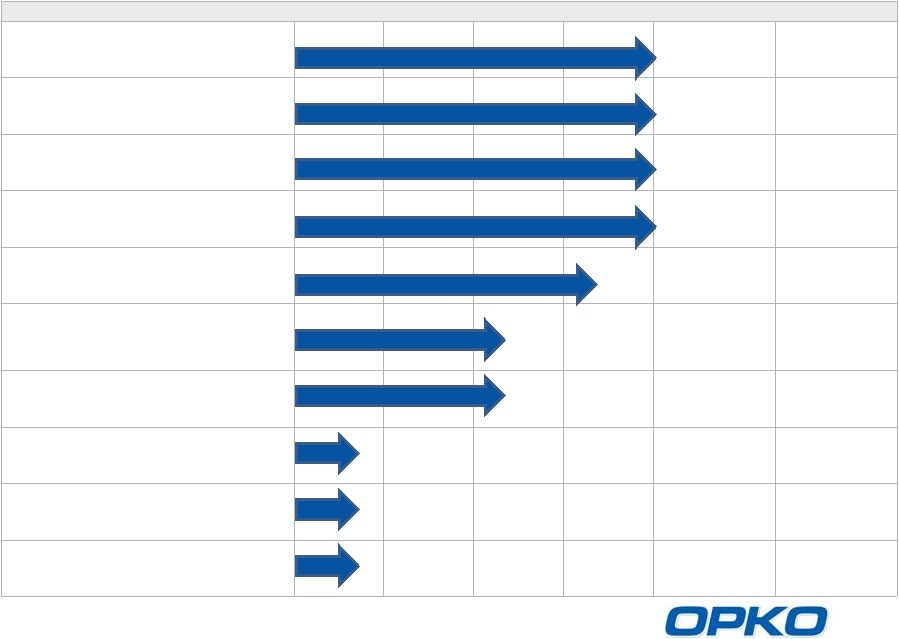
OPKO
Pharmaceuticals Advanced, Deep Pipeline
Product
Indication
Preclinical
Phase 1
Phase 2
Phase 3
Milestone
Market Size
Rayaldee
(CTAP101)
SHPT
(CKD Stage 3-4 Patients)
Phase 3 results
reported
3Q 2014
$12.0 BN
hGH-CTP
hGH deficiency
$3.5 BN
Alpharen
(Fermagate)
Hyperphosphatemia
(CKD Stage 5 Patients)
$1.2 BN
Rolapitant
CINV
NDA accepted
November, 2014
$1.5 BN
Sci-B-Vac
Hepatitis B
(CKD Stage 5 Patients)
$0.2BN
Lunacalcipol
(CTA018)
Moderate to severe
SHPT
(CKD Stage 5 Patients) &
Psoriasis
$1.5 BN
CTAP201
Mild to moderate SHPT
(CKD Stage 5 Patients)
$1.1 BN
Factor VIIa-CTP
Hemophilia
$1.7 BN
AntagoNAT
Platform
Cancer, CV, metabolic
and orphan disease
$1.0 BN
Oxyntomodulin
Diabetes, Obesity
$15 BN
Collaboration with Pfizer
Outlicensed to TESARO
8 |

Rayaldee (CTAP101)
A Late-Stage Investigational Drug
Product Overview
Oral formulation of 25D
3
*
addresses significant unmet
need
Achieves reliable increases in
serum 25D and reductions in
plasma PTH in Stage 3-4 CKD
Lower risk of side effects
compared to active 1,25D
**
products
Phase 1 cancer trail begun
Clinical Status
Intellectual Property
* 25-Hydroxyvitamin
D3
**
1,25-Dihydroxyvitamin D
Clinical development guided
by prominent Scientific
Advisory Board
Positive Phase 3 data
NDA submission target:
1Q2015
Rayaldee US patents issued,
protected through 2028
Additional global patents
allowed or pending
9 |
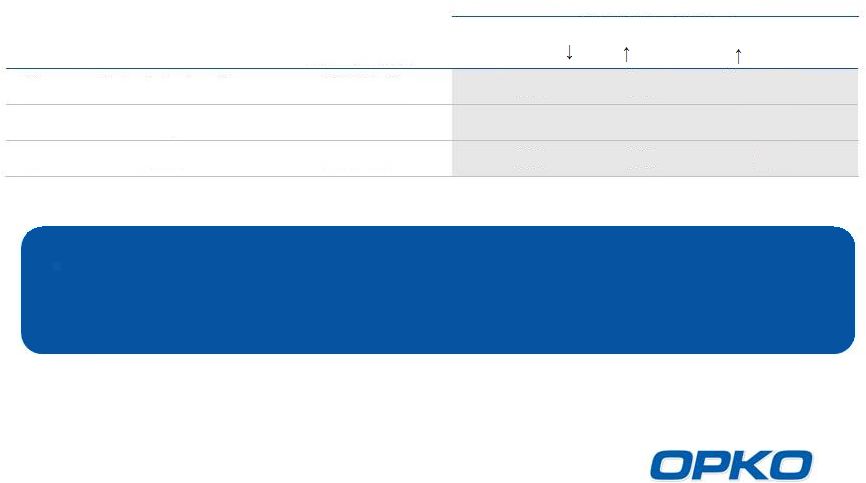
Market Opportunity: Chronic Kidney Disease (U.S.)
*US Renal Data Service 2013 Annual Data Report
Sources: Levin, A et al., Kidney International 2007; 71:
pp.31-38.
Gonzalez, E et al. Am J Nephrol 2004;24:503-510.
LaClair, R et al. Am J Kidney Dis 2005;45:1026-1033.
The CKD patient population is large and growing as a result of:
Obesity
Hypertension
Diabetes
Over half of the CKD population can be reached with a small
sales force targeting nephrologists and endocrinologists
10
% of CKD Patients with:
Stage
Kidney Function
CKD Prevalence
Vitamin D
Insufficiency( 25D)
SHPT
( PTH)
Hyperphosphatemia
( Phosphorus)
3
Moderate impairment
18.7
Million*
70%
56%
37%
4
Severe impairment
1.4 Million*
80%
60%
50%
Failure
0.5 Million*
90%
90%
70%
5 |
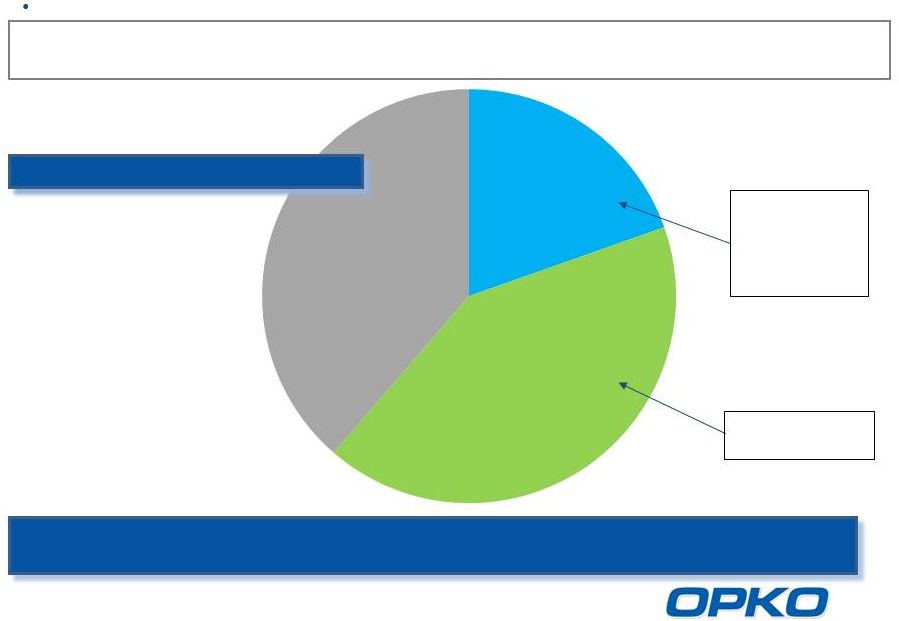
Rayaldee -
Commercial Opportunity
Source: BioTrends Research Group, Inc. December 2013
Untreated
26-44%
Vitamin D
Hormone
14-25%
Nutritional
Vitamin D
39-44%
Untreated
31-47%
Safety
concerns;
exacerbates
vitamin D
insufficiency
Efficacy
Concerns
Stage 3 & 4 CKD Treatment
11
Low
serum
25D
and
elevated
plasma
PTH
are
prevalent
in
CKD
Stage
3-4
patients
20 million CKD Stage 3-4 patients in the US
8 million patients with low serum 25D and high plasma PTH
Rayaldee is expected to take significant market share in Stage 3 and 4 CKD
patients suffering from SHPT a potential $12 billion revenue
opportunity
|
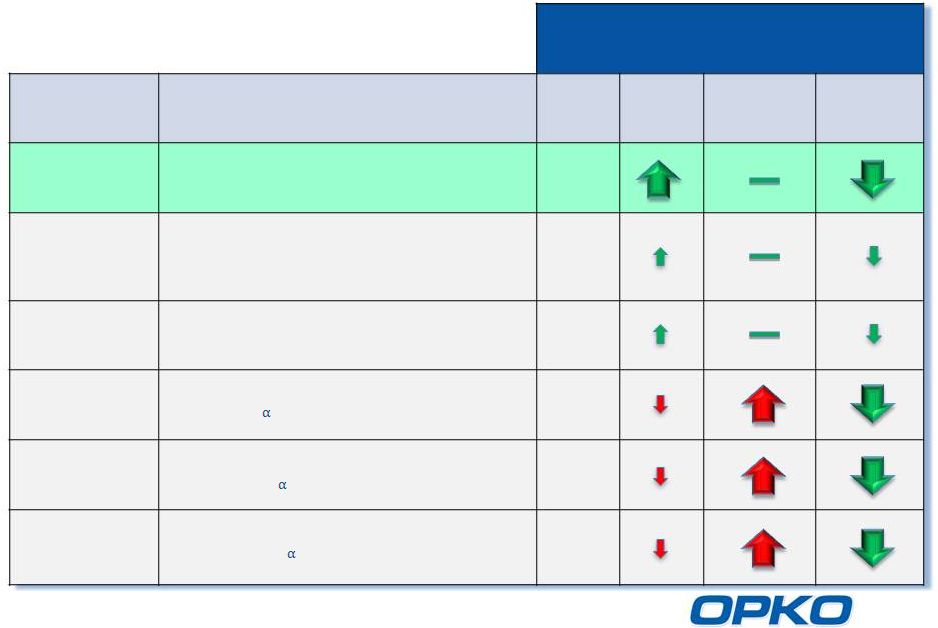
Comparison of Vitamin D Therapies for Stage 3-4 CKD
*And generics
**25-hydroxyvitamin D
12
Drug
Active
Type
25D
**
Ca
iPTH
Rayaldee
Calcifediol
(25-hydroxyvitamin D
3
)
Rx
Vitamin D
Cholecalciferol/Ergocalciferol
(vitamin D
3
/vitamin D
2
)
OTC
Rx
Rx
Rx
Rx
Drisdol
*
Ergocalciferol
(vitamin D
2
)
Rocaltrol
*
Calcitriol
(1 ,25-dihydroxyvitamin D
3
)
Hectorol
*
Zemplar
*
Paricalcitol
(19-nor-1 ,25-dihydroxyvitamin D
2
)
Doxercalciferol
(1 -hydroxyvitamin D
2
)
Effect on Blood Levels of: |
OPKO Biologics: Leader in BioBetter Drugs
Reversible Pegylation Technology
(Peptides and small molecules)
CTP Technology
(Recombinant Proteins)
Developing biobetter long acting proteins and peptides
Dramatically reduce injection frequency
Reduce drug load
Reduce potential side-effects for most proteins, peptides and
small molecules
Maintain drug bio-activity
Validated
platform
technologies
safe
and
effective
-
Preclinical & clinical proof-of-concept in multiple compounds
13 |
Natural sequence
No need for linker
hGH-CTP
The Long Acting hGH
14
+
=
Any Short-Lasting
Protein
CTP
Long-Lasting Protein |
Global Collaboration with Pfizer for OPKOs Long-
Acting Human Growth Hormone (hGH-CTP)
15
Collaboration Terms:
$295 M up-front payment
$275 M for achievement of regulatory based milestones
OPKO responsible for funding development program for the key
indications:
Adult and Pediatric Growth Hormone Deficiency (GHD)
Pediatric Short for Gestational Age
Pfizer responsible for funding:
Development programs for additional indications
All Post Marketing Studies
All Commercialization Activities
Initial double digit tiered royalties on sales of Adult GHD
Profit sharing commencing upon launch for Pediatric GHD
encompassing combined sales for all indications of OPKOs hGH-CTP
and Pfizers Genotropin
Pfizer Genotropin represents about 25% of the world market with
annual revenues exceeding $700 M
Financial
Commercial
Development |
|
$3.5 billion market, growing 5% annually
Once-a-week injection (current products require daily injections)
Small needle size (31 gauge) due to low viscosity
Competitive long acting formulations have high viscosity
Superb clinical, safety and immunogenicity profile
Human growth hormone is used for:
Growth hormone deficient children
Growth hormone deficient adults
Short stature
Off label
Orphan drug designation in the US & EU for children & adults
hGH-CTP Opportunity
16 |
| hGH-CTP Clinical Development
Adult Pivotal Phase 3 trial (ongoing)
Primary efficacy endpoint: reduction in truncal fat mass
after 6 months vs. placebo
Secondary efficacy endpoints include:
Reduction in total body fat
Increase in lean body mass
Single pivotal trial required by FDA for BLA submission
17 |
| hGH-CTP Clinical Development
Pediatric GHD Phase 2 trial (advanced stage)
4 Cohorts:
3 dose levels of once-weekly hGH-CTP
Commercially available standard daily rhGH treatment
Key outcome: height velocity
Positive clinical data presented at ENDO meeting June 2014
All doses provided excellent growth response compared to control
group and historical controls
Promising safety profile
hGH-CTP mean annualized height velocity at 6m ranged from 12.25-
14.37cm, compared to annual height velocity of ~10cm as published by
Bakker (2008) and Ranke (2010) for the same GHD patient population
(peak GH, age)
18 |
| FVIIa
CTP:
Long Acting for Treating Hemophilic Patients
$1.7 billion market
Growing 7% annually
Only 25% of patients are treated
Current product (NovoSeven®) requires frequent IV doses
3-4 times a day during bleeding episodes
1-2 times a day for prophylactic treatment
Pharmacological
studies
in
hemophilic
mice
and
dogs
FVIIa-CTP
demonstrated:
Reduce injection frequency during on-demand therapy
Enable prophylactic treatment while reducing the frequency of
injections to 2-3 times a week
Potential for substantial improvement in the quality of life of patients via
subcutaneous administration
Phase 2a study in hemophilic patients to be initiated 1Q 2015
Orphan drug designation in the US and EU
19 |
| MOD-6031: Long Acting Oxyntomodulin for Obesity
WHO estimates 500 million severely overweight or obese people
>$15 billion market -
growing rapidly
Oxyntomodulin: Natures Appetite Control Mechanism
Natural appetite suppressor
Secreted by the digestive system following food intake and induces
satiety
Increases glucose tolerance
Short acting
requires 3 injections per day
MOD-6031 Long Acting Oxyntomodulin-
weekly injection studies in
mutant obese mice and diet induced obese mice demonstrated:
Significantly inhibited food intake and reduced body weight by
reducing fat
Reduced cholesterol levels
Improved glycemic control
Phase 1 study to be initiated mid 2015
MOD-6031 is expected to provide SAFE long-term therapy for
obesity and diabetes type II patients
20 |
| Rolapitant
Potential Near-term Revenue Driver
Rolapitant out-licensed to Tesaro in December 2010
Payments of up to $121 million
Double-digit tiered royalties
Differentiated cancer supportive care product with $1.5B US Market
Opportunity
Potent neurokinin-1(NK-1) receptor antagonist for
chemotherapy- induced nausea and vomiting (CINV)
Opportunity to differentiate on convenience, market access and safety
Single dose
Long acting
NDA accepted November 2014
All three Phase 3 trials (MEC* and HEC**) achieved primary endpoint
Primary endpoint: complete response (no emesis and no use of rescue
medication)
Third Phase 3 trial (HEC) also achieved all secondary endpoints,
including:
Complete response in acute (0-24 hrs) and overall (0-120 hrs) phase of
CINV
Also effective to prevent nausea
21
*Moderately emetogenic chemotherapy **Highly emetogenic
chemotherapy
Oral and IV formulations allow full market access
Lack of drug-drug interactions |
| Strategic Investments
ARNO Therapeutics, Inc. (OTC: ARNI) (~4% equity interest)
Anti-progestins for breast (phase 2), endometrial and prostate cancers
Zebra Biologics, Inc. (~19% equity interest)
Combinatorial
antibody
libraries
based
on
function
in
human
cell
screens
OAO Pharmsynthez (MICE: LIFE) (~17% equity interest)
Russian developer and marketer of new drugs
RXi Pharmaceuticals Corporation (NASDAQ: RXII) (~11% equity interest)
sRNA to prevent hypertrophic scars (phase 2)
Cocrystal Pharma, Inc. (OTC: COCP) (~15% equity interest)
New anti-virals (Hepatitis C, flu, dengue fever)
Sevion Therapeutics, Inc. (OTC: SVON) (~ 4% equity interest*)
Antibodies against difficult targets (e.g., G protein-coupled receptor, ion
channels)
Neovasc, Inc. (NASDAQ: NVCN) (~ 6% equity interest)
Cardiology devices
ChromaDex, Inc. (OTC: CDXC) (~ 2% equity interest)
New nutritional supplement APIs
22
Proprietary Technologies with Significant Upside Potential
(As of September 30, 2014) |