EX-99.1
Published on June 18, 2015
Exhibit 99.1
|
|
Exhibit 99.1
Confidential
Update on
Rayaldee Capsules
June 18, 2015
|
|
Cautionary Statement
This presentation contains forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA), which statements may be identified by words such as expects, plans, projects, will, may, anticipates, believes, should, intends, estimates,
potential and other words of similar meaning, including statements regarding our estimated revenues and financial projections, our ability to achieve high levels of growth, the potential for our products under development, the potential of the 4Kscore to reduce prostate biopsies and predict the risk of aggressive prostate cancer, our ability to develop, test and launch new products, the expected timing of the clinical studies and regulatory submissions relating to our products under development, the outcome of our clinical trials and validation studies and that such outcomes will support commercialization, the expected market penetration and size of the market for our products under development, including without limitation, Rolapitant, Rayaldee (CTAP-101), hGH-CTP, the 4Kscore, Factor VIIa-CTP, oxyntomodulin, and our point-of-care diagnostic products for Total-PSA, testosterone, and Vitamin D, the potential benefits of our products under development, including whether the 4Kscore will improve selection of candidates for prostate biopsy, predict the risk of distant metastases, and result in $2 to 4 billion in healthcare savings, expected per patient savings, whether MOD-6031 will provide superior long-term therapy for obesity and Type II diabetes patients, our ability to successfully commercialize our product candidates such as Rolapitant, the 4Kscore, Rayaldee (CTAP-101), hGH-CTP, Factor VIIa-CTP, and oxyntomodulin, as well as products for other markets such as urology, womens health, cardiology, oncology, iPTH, and infectious disease, whether we will be able to develop Rayaldee (CTAP-101) for additional indications and whether Rayaldee (CTAP-101) will take significant market share in Stage 3 and 4 CKD patients with SHPT, whether Rayaldee (CTAP-101) will raise serum total 25-hydroxyvitamin D (25D) more effectively than any over-the-counter (OTC) or prescription (Rx) product currently marketed without the risk of hypercalcemia, whether we can reach more than half of the CKD population with a small sales force, our ability to establish a sales and marketing and clinical support infrastructure for Rayaldee and the timeline for doing so, the expected acceptance date of our Rayaldee NDA and PDUFA date, expectations regarding patent coverage, the expected timing for commencing, completing and obtaining results for our clinical trials and publication date for NCCN guidelines, the timing for release of trial data and seeking and obtaining FDA and European regulatory approvals as well as reimbursement coverage, and the timing of commercial launch of our product candidates, as well as other non-historical statements. These forward-looking statements are only predictions and reflect our views as of the date they were made, and we undertake no obligation to update such statements. Such statements are subject to many risks and uncertainties that could cause our activities or actual results to differ materially from the activities and results anticipated in forward-looking statements, including risks inherent in funding, developing and obtaining regulatory approvals of new, commercially-viable and competitive products and treatments, the success of our collaboration with Pfizer, general market factors, competitive product development, product availability, federal and state regulations and legislation, and integration issues arising from the transactions, delays associated with development of novel technologies, unexpected difficulties and delays in validating and testing product candidates, the regulatory process for new products and indications, manufacturing issues that may arise, the cost of funding lengthy research programs, the need for and availability of additional capital, the possibility of infringing a third partys patents or other intellectual
property rights, the uncertainty of obtaining patents covering our products and processes and in successfully enforcing them against third parties, and the possibility of litigation, among other factors, including all of the risks identified under the heading Risk Factors in our Annual Report on Form 10-K and other filings with the Securities and Exchange Commission.
2
|
|
Rayaldee Initial Indication Requested in New Drug Application
Prevention and treatment of secondary hyperparathyroidism (SHPT) in patients with stage 3 or 4 chronic kidney disease
(CKD) and vitamin D insufficiency
Significance: First drug to target this important indication
2
|
|
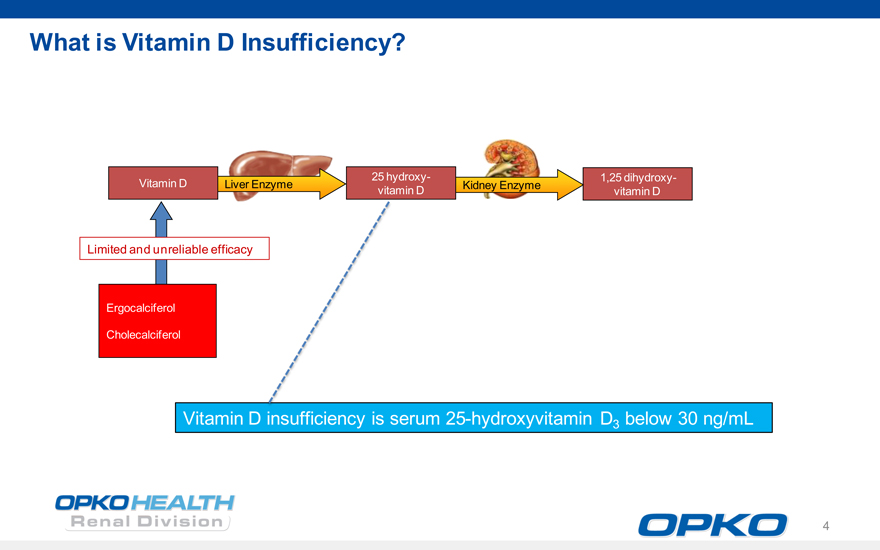
What is Vitamin D Insufficiency?
25 hydroxy- 1,25 dihydroxy- Vitamin D Liver Enzyme Kidney Enzyme vitamin D vitamin D
Limited and unreliable efficacy
Ergocalciferol
Cholecalciferol
Vitamin D insufficiency is serum 25-hydroxyvitamin D3 below 30 ng/mL
4
|
|
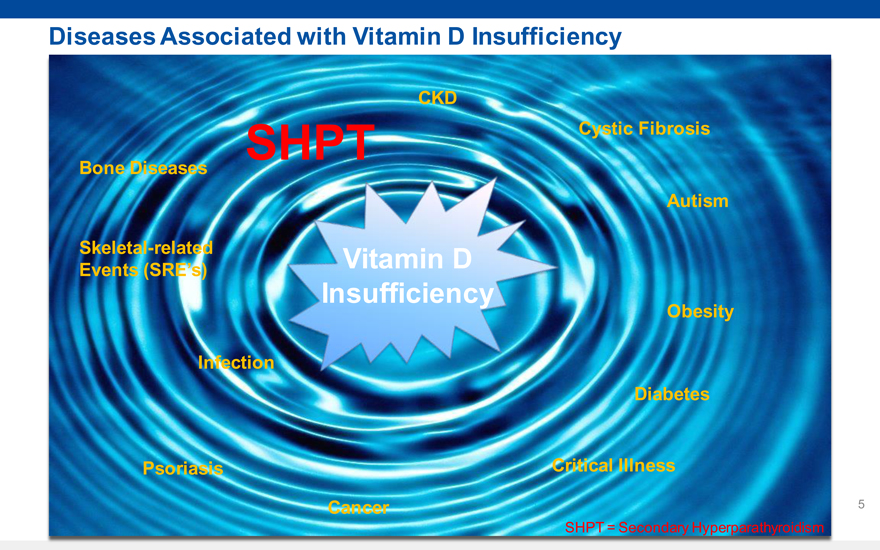
Diseases Associated with Vitamin D Insufficiency
CKD
SHPT Cystic Fibrosis
Bone Diseases
Autism
Skeletal-related Vitamin D
Events (SREs)
Insufficiency
Obesity
Infection
Diabetes
Psoriasis Critical Illness
Cancer
5
SHPT = Secondary Hyperparathyroidism
|
|
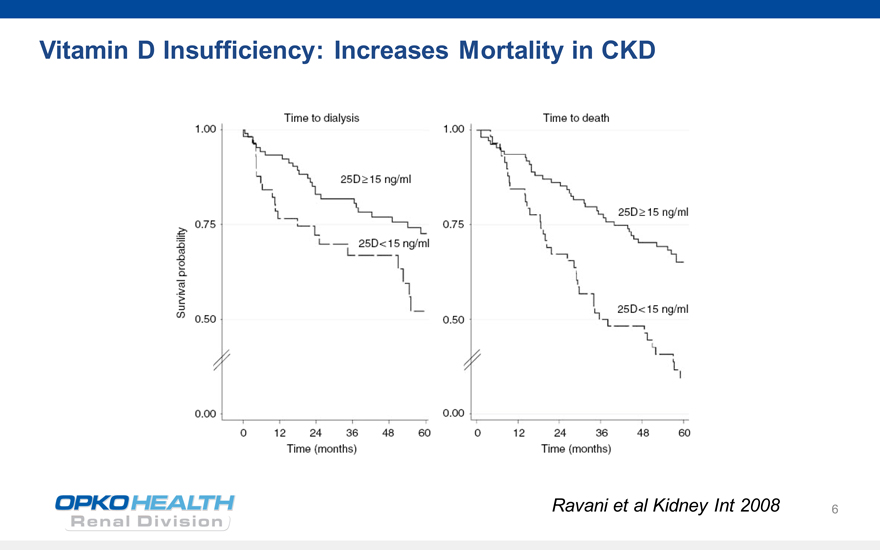
Vitamin D Insufficiency: Increases Mortality in CKD
1.0 Time to dialysis 25D?15 ng/ml
0.75 25D?15 ng/ml 25D<15 ng/ml
0.75 0.50 0.00
Survival probability
0
12
24
36
48
60
Time (months)
1.00
Time to dealth
0.75
25D?15 ng/ml
0.50
25D<15 ng/ml
0.00
0
12
24
36
48
60
Time (months)
Ravani et al Kidney Int 2008 6
|
|
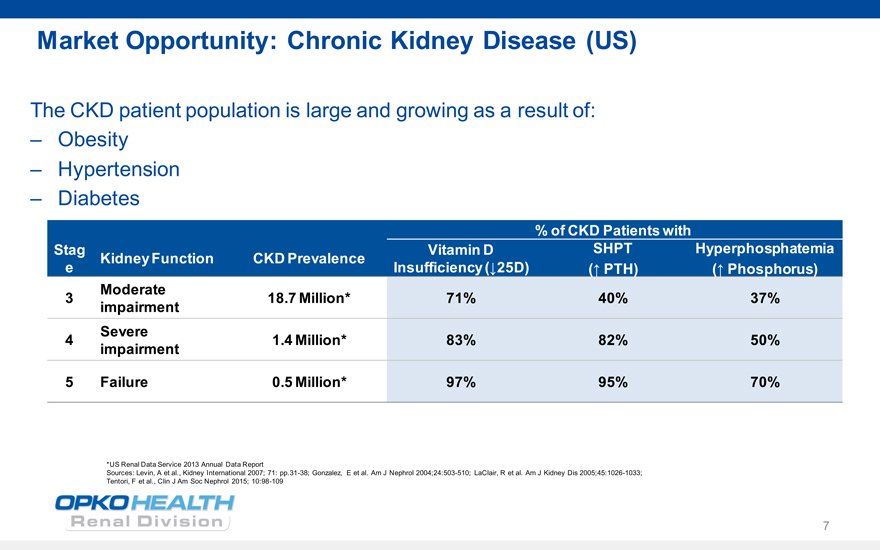
Market Opportunity: Chronic Kidney Disease (US)
The CKD patient population is large and growing as a result of:
Obesity
Hypertension
Diabetes
% of CKD Patients with
Stag Vitamin D SHPT Hyperphosphatemia Kidney Function CKD Prevalence e Insufficiency (?25D) (? PTH) (? Phosphorus)
Moderate
3 18.7 Million* 71% 40% 37% impairment Severe
4 1.4 Million* 83% 82% 50% impairment
5 Failure 0.5 Million* 97% 95% 70%
*US Renal Data Service 2013 Annual Data Report
Sources: Levin, A et al., Kidney International 2007; 71: pp.31-38; Gonzalez, E et al. Am J Nephrol 2004;24:503-510; LaClair, R et al. Am J Kidney Dis 2005;45:1026-1033; Tentori, F et al., Clin J Am Soc Nephrol 2015; 10:98-109
7
|
|
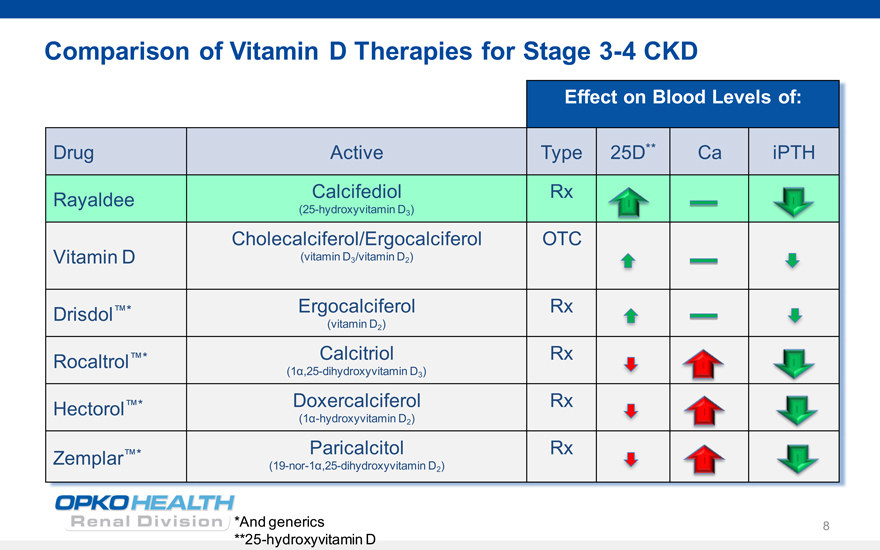
Comparison of Vitamin D Therapies for Stage 3-4 CKD
Effect on Blood Levels of:
Drug Active Type 25D** Ca iPTH
Rayaldee Calcifediol Rx
(25-hydroxyvitamin D3)
Cholecalciferol/Ergocalciferol OTC
Vitamin D (vitamin D3/vitamin D2)
Drisdol* Ergocalciferol Rx
(vitamin D2)
Rocaltrol* Calcitriol Rx
(1?,25-dihydroxyvitamin D3)
Hectorol* Doxercalciferol Rx
(1?-hydroxyvitamin D2)
* Paricalcitol Rx Zemplar
(19-nor-1?,25-dihydroxyvitamin D2)
*And generics **25-hydroxyvitamin D
8
|
|
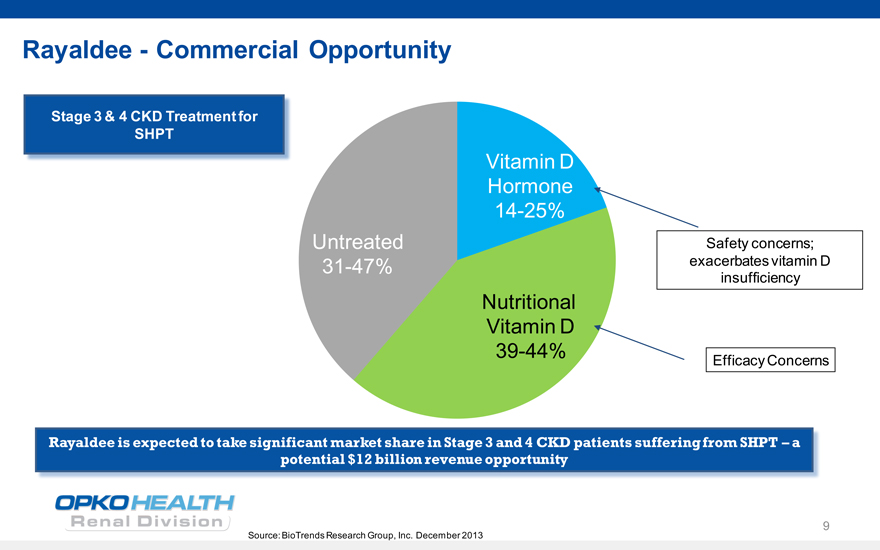
RayaldeeCommercial Opportunity
Stage 3 & 4 CKD Treatment for SHPT
Vitamin D Hormone 14-25%
Untreated Safety concerns; 31-47% exacerbates vitamin D insufficiency
Nutritional Vitamin D
39-44%
Efficacy Concerns
Rayaldee is expected to take significant market share in Stage 3 and 4 CKD patients suffering from SHPT a potential $12 billion revenue opportunity
Source: BioTrends Research Group, Inc. December 2013
9
|
|
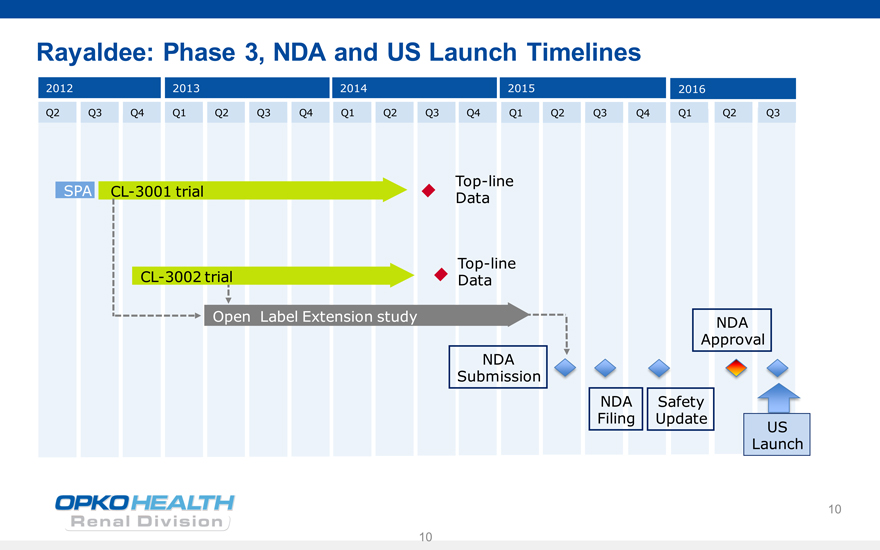
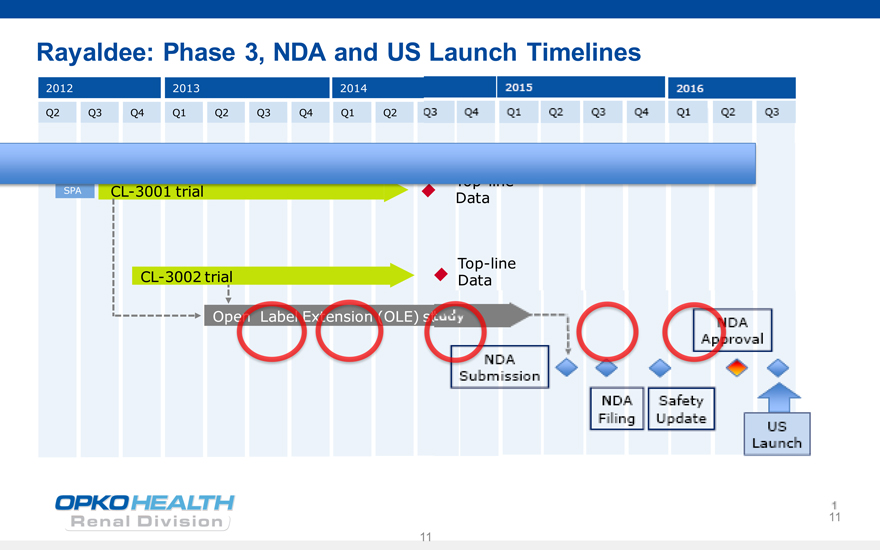
Rayaldee: Phase 3, NDA and US Launch Timelines
2012 2013 2014 2015 2016
Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3
Top-line SPA CL-3001 trial Data
Top-line CL-3002 trial Data
Open Label Extension study NDA Approval NDA
Submission
NDA Safety Filing Update
US Launch
10
|
|
Rayaldee: Phase 3, NDA and US Launch Timelines
2012 2013 2014
Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2
Data
Top-line CL-3002 trial Data Ope (OLE)
11
|
|
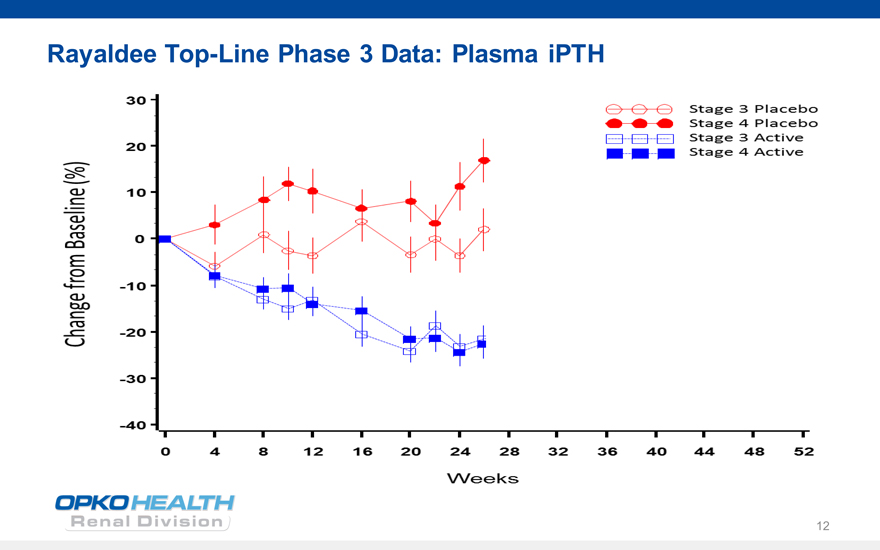
Rayaldee Top-Line Phase 3 Data: Plasma iPTH
Change from Baseline (%)
30
20
10
0
10
20
30
40
0
4
8
12
16
20
24
28
32
36
40
44
48
52
Weeks
Stage 3 placebo
Stage 4 plaebo
Stage 3 Active
Stage 4 Active
12
|
|
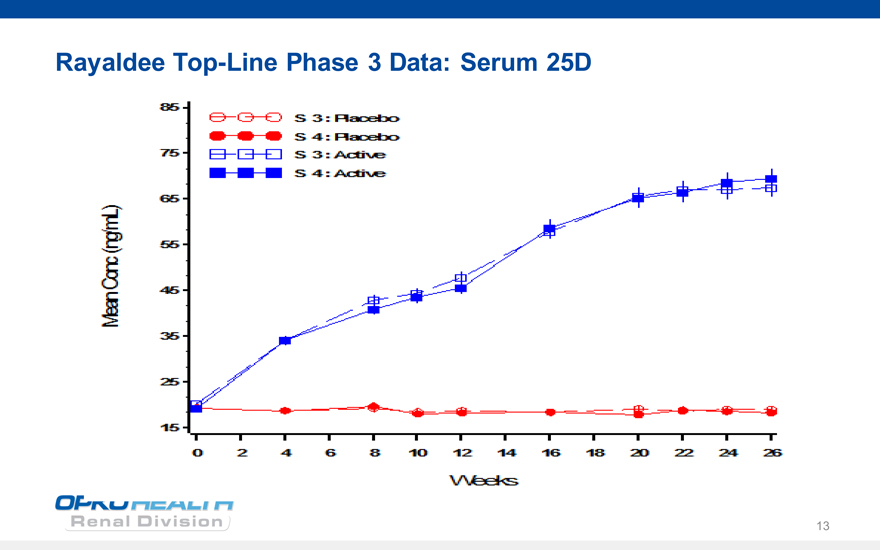
Rayaldee Top-Line Phase 3 Data: Serum 25D
85
75
65
55
45
35
25
15
0
1
2
4
6
8
10
12
14
16
18
20
22
24
26
Meen Conc(ngml)
13
|
|
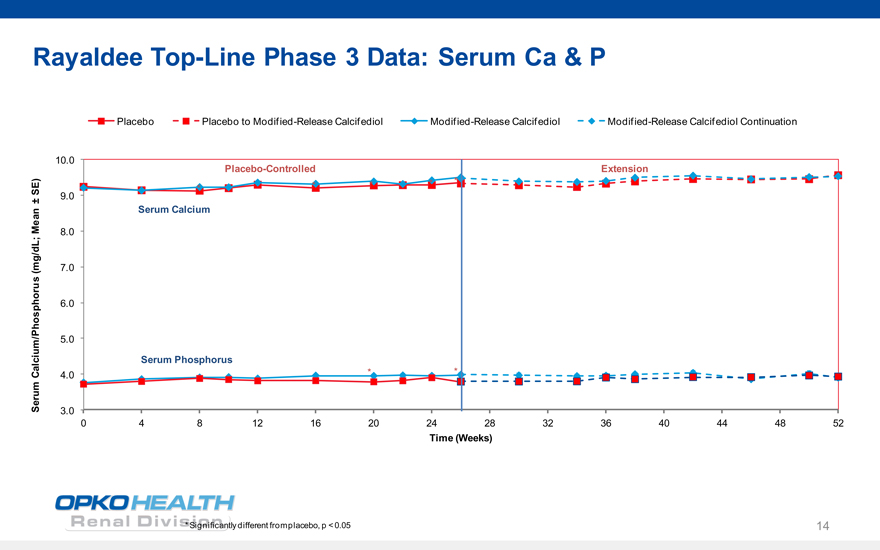
Rayaldee Top-Line Phase 3 Data: Serum Ca & P
Placebo Placebo to Modified-Release Calcifediol Modified-Release Calcifediol Modified-Release Calcifediol Continuation
10.0 Placebo-Controlled Extension *
* * *
SE) *
± 9.0
Mean Serum Calcium
8.0
(mg/dL; 7.0
6.0
5.0
Serum Phosphorus
Calcium/Phosphorus 4.0 * * Serum
3.0
0 4 8 12 16 20 24 28 32 36 40 44 48 52
Time (Weeks)
* Significantly different from placebo, p < 0.05
14
|
|
Rayaldee Key Steps to Commercialization
NDA filing (accepted for full FDA review) in late July
Marketing & sales management team to be hired in late 2015
Sales representatives and clinical support specialists to be hired pre-launch
PDUFA date: late May 2016
Launch expected within 3 months of PDUFA date
Initial line extension plans:
Additional phase 3 clinical trial(s) planned in stage 5 CKD
Phase 1 clinical trial ongoing for new oncology indication
Other indications being evaluated
14
|
|
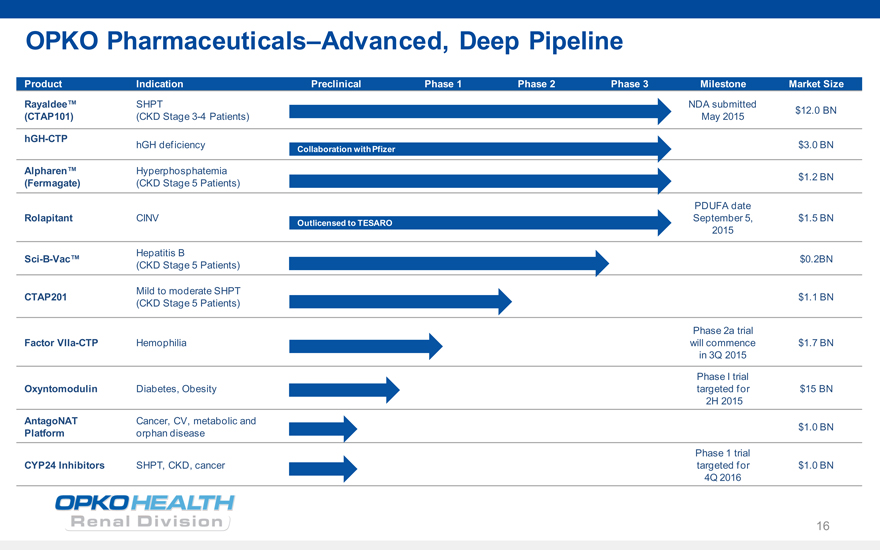
OPKO PharmaceuticalsAdvanced, Deep Pipeline
Product Indication Preclinical Phase 1 Phase 2 Phase 3 Milestone Market Size
Rayaldee SHPT NDA submitted
$12.0 BN
(CTAP101) (CKD Stage 3-4 Patients) May 2015
hGH-CTP hGH deficiency $3.0 BN
Collaboration with Pfizer
Alpharen Hyperphosphatemia
$1.2 BN
(Fermagate) (CKD Stage 5 Patients)
PDUFA date
Rolapitant CINV September 5, $1.5 BN
Outlicensed to TESARO
2015
Hepatitis B
Sci-B-Vac $0.2BN
(CKD Stage 5 Patients)
Mild to moderate SHPT
CTAP201 $1.1 BN
(CKD Stage 5 Patients)
Phase 2a trial
Factor VIIa-CTP Hemophilia will commence $1.7 BN in 3Q 2015
Phase I trial
Oxyntomodulin Diabetes, Obesity targeted for $15 BN
2H 2015
AntagoNAT Cancer, CV, metabolic and
$1.0 BN
Platform orphan disease
Phase 1 trial
CYP24 Inhibitors SHPT, CKD, cancer targeted for $1.0 BN
4Q 2016
16
|
|
Thanks!
17