EX-99.3
Published on June 18, 2015

General
Overview 18 June 2015
Exhibit 99.3 |

Our Vision We are a leading specialty pharmaceutical company To be the leading developer and supplier of highly potent pharmaceutical products for global markets Click above to view EirGens expanded facility |
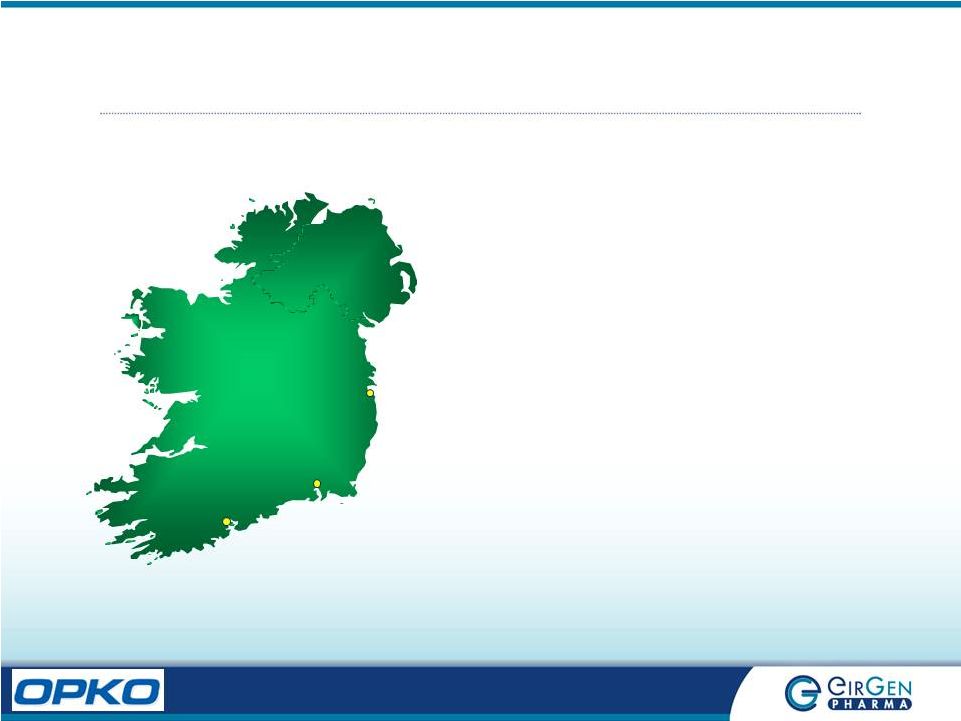
Ireland:
Fact Sheet Waterford
Cork Dublin Population 4.2 million Largest Exporter of Pharmaceuticals in the World, 8 th largest producer. 9 of Worlds Top 10 Pharma Companies Located in Ireland Home to 120 Pharma companies; 33 pharma and biopharma plants are FDA approved. 25,000 direct & 25,000 indirect employees serviced by excellent university programs English speaking and excellent compliance track record Waterford is situated in the South East of Ireland. Population of 50,000 people. |
Business
Overview A Specialty
Pharmaceutical Company
Focused on the development and
commercial supply of highly potent
pharmaceutical products
In a purpose built, state of the art,
high containment facility |

Introductions EirGen Founders PATSY CARNEY Chief Executive Officer/ Co-Founder » Co-founded EirGen » Previously Head of Operations and BD for IVAX Ireland (14 years) » Holds a BSc In Industrial Chemistry from University of Limerick and an MBA from University of Limerick TOM BRENNAN Chief Technical Officer/ Co-Founder » Co-Founded EirGen » Previously held senior technical positions with IVAX and Stada » 10 Years IVAX Ireland as R&D Manager » Holds a BSc in Chemistry from Cork IT, an MSc in Industrial Pharmacy from University of Manchester and an MBA from University of Limerick » Qualified Person, Six Sigma Black Belt, Packaging Technologist |
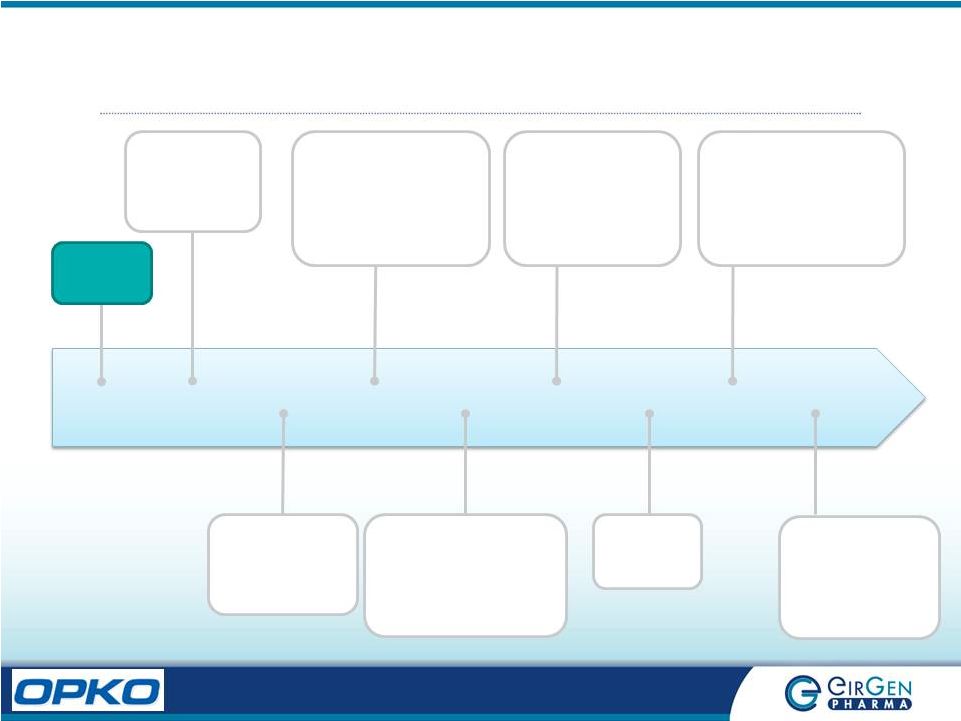
EirGen
Key Milestones 2005
2006 2008 2009 2011 2012 2013 Successful Regulatory Audit OPEN FOR BUSINESS Submission of First Pan EU EirGen License 1. 1 st License Approval in Canada 2. 5 th Oncology product license submitted to US FDA 3. 1 st commercial product launch in EU 1. Two oncology product licenses submitted to Japanese PMDA 2. US FDA facility approval and product approval 1. Japanese PMDA facility approval and 1 st product approval 2. US FDA Inspection, zero 483s EirGen Founded Facility expansion complete 2014 1. US FDA Inspection, zero 483s 2. Soft-gel fitout complete 3. 31 st clinical study complete and passed 4. 10 th ANDA submitted to US FDA 2015 1. 5 th IMB (EMA) Inspection no major observations 2. Acquisition by OPKO Health |

Current
Position: where we are now »
98 employees, all graduates
» 31 successful Clinical Studies » 42 countries for commercial product supply » 27 products in R&D » 10 Products filed with US FDA to date, 3 approved » 4 Pan EU CTD Dossiers filed to date and approved » 5 Oncology Products filed with Japanese PMDA; 4 approved |

Current
Position: Regulatory Status »
Global license submission strategy
» FDA, Aug 2014, no 483s » IMB (EMA), May 2015, no major observations » Licensed to handle Cytotoxic Materials |

Therapeutic Focus » Oncology Molecules » Immunosuppressants » Prostaglandins » Cytotoxic Molecules » Hormonals » Steroids |
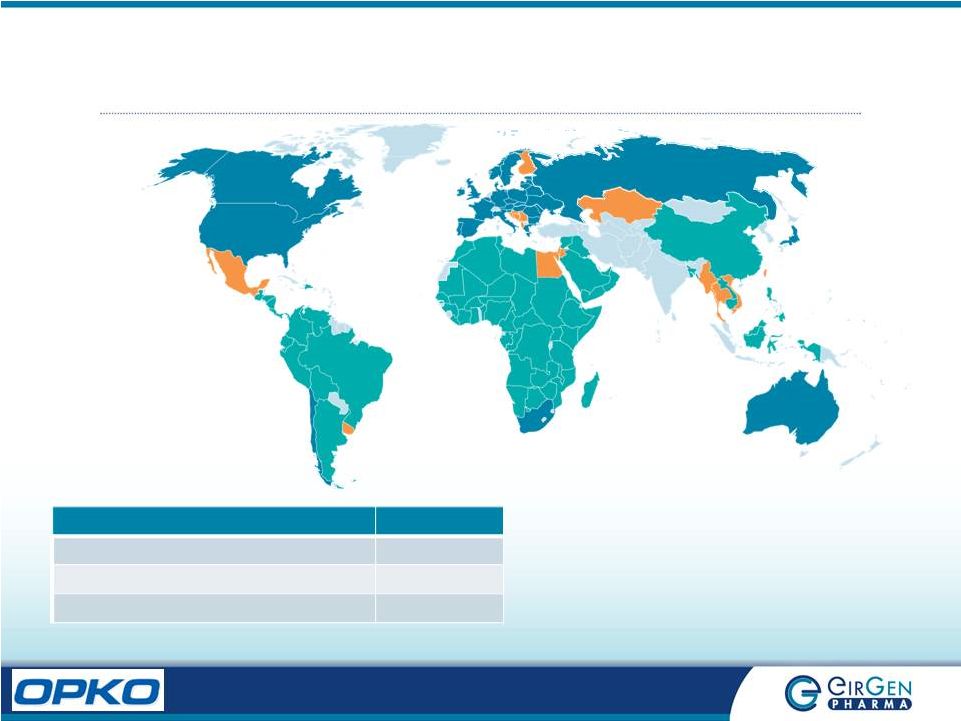
7 Global Presence Category Countries Commercial (BLUE) 42 Licenses Pending (ORANGE) 20 Customer Contracts (GREEN) 85 |

EirGen
Existing Business Model R&D (non-
GMP and GMP) Clinical studies and license approval Routine on- going commercial supply Sales, marketing & distribution Strategic Partner |
EirGen
OPKO Opportunities »
API Vertical Integration - Synergies with OPKO Finetech, Israel » Product Development & Manufacturing - OPKOs current & future products » Irish based Supply Chain Hub - global distribution of OPKO Products » Tax efficient R&D Hub - proven R&D track record since incorporation |

Thank
You www.EirGen.com |