EX-10.27
Published on July 28, 2011
CONFIDENTIAL
MATERIAL OMITTED AND FILED
SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
ASTERISKS DENOTE SUCH OMISSIONS.
SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
ASTERISKS DENOTE SUCH OMISSIONS.
Exhibit 10.27
EXCLUSIVE LICENSE AGREEMENT
BY AND BETWEEN
TESARO, INC.
AND
OPKO HEALTH, INC.
Table of Contents
| Page | ||||
Article I DEFINITIONS |
1 | |||
Article II LICENSE GRANT |
8 | |||
2.1 License Grant |
8 | |||
2.2 Sublicenses |
8 | |||
2.3 Covenant Not to Sue |
9 | |||
2.4 Responsibility; Decision-making |
9 | |||
2.5 Diligence |
9 | |||
2.6 Joint Steering Committee |
10 | |||
2.7 Latin America |
10 | |||
Article III TECHNOLOGY TRANSFER AND TRANSITION ACTIVITIES |
11 | |||
3.1 Know-how Transfer |
11 | |||
3.2 Cooperation |
11 | |||
3.3 Regulatory Transition |
11 | |||
3.4 Supply of Material |
12 | |||
3.5 Costs |
12 | |||
Article IV FINANCIAL PROVISIONS |
12 | |||
4.1 License Fee |
12 | |||
4.2 Intentionally Left Blank |
12 | |||
4.3 Milestones Payments by TESARO |
12 | |||
4.4 Royalty Payments by TESARO |
13 | |||
4.5 Japan |
15 | |||
4.6 Royalty and Income Sharing Term |
15 | |||
4.7 Reduction for No Valid Claim |
15 | |||
4.8 Third Party Payments |
16 | |||
4.9 Payments; Reports |
16 | |||
4.10 Taxes |
16 | |||
4.11 United States Dollars |
17 | |||
4.12 Currency Conversion |
17 | |||
4.13 Blocked Payments |
17 | |||
4.14 Late Payments |
17 | |||
4.15 Records and Audits |
17 | |||
Article V INTELLECTUAL PROPERTY OWNERSHIP, PROTECTION AND RELATED
MATTERS |
18 | |||
5.1 Prosecution and Maintenance of Patent Rights |
18 | |||
5.2 Patent Term Extensions |
19 | |||
5.3 Third Party Infringement |
19 | |||
5.4 Patent Invalidity Claim |
20 | |||
5.5 Patent Marking |
21 | |||
Article VI CONFIDENTIALITY |
21 | |||
6.1 Confidential Information |
21 | |||
i
| Page | ||||
6.2 Permitted Disclosures |
22 | |||
6.3 Limitation on OPKO Disclosure of OPKO Know-how |
22 | |||
6.4 Publicity |
22 | |||
6.5 Publications |
23 | |||
6.6 Return of Confidential Information |
23 | |||
Article VII REPRESENTATIONS AND WARRANTIES; CERTAIN COVENANTS |
23 | |||
7.1 Mutual Representations |
23 | |||
7.2 OPKOs Representations and Warranties |
24 | |||
7.3 No Warranty |
25 | |||
Article VII. INDEMNIFICATION |
25 | |||
8.1 Indemnification by TESARO |
25 | |||
8.2 Indemnification by OPKO |
26 | |||
8.3 Indemnification Procedure |
26 | |||
8.4 Limitation of Liability |
26 | |||
8.5 Insurance |
27 | |||
Article IX TERM AND TERMINATION |
27 | |||
9.1 Term |
27 | |||
9.2 Termination for Convenience |
27 | |||
9.3 Termination for Cause |
27 | |||
9.4 OPKO Termination |
27 | |||
9.5 Effect of Termination |
27 | |||
9.6 Survival |
29 | |||
Article X DISPUTE RESOLUTION |
29 | |||
10.1 Continuance of Rights and Obligations During Pendency of
Dispute Resolution |
29 | |||
10.2 Referral of Unresolved Matters to Senior Executives |
29 | |||
10.3 Arbitration |
29 | |||
10.4 Equitable Relief |
30 | |||
Article XI MISCELLANEOUS |
30 | |||
11.1 Governing Law and Jurisdiction |
30 | |||
11.2 Force Majeure |
30 | |||
11.3 Further Assurances |
31 | |||
11.4 Notices |
31 | |||
11.5 Assignment |
31 | |||
11.6 Affiliate Performance |
32 | |||
11.7 Amendment |
32 | |||
11.8 Entire Agreement |
32 | |||
11.9 No Benefit to Third Parties |
32 | |||
11.10 Waiver |
32 | |||
11.11 No Implied Licenses |
32 | |||
11.12 Relationship of the Parties |
32 | |||
11.13 Severability |
32 | |||
ii
| Page | ||||
11.14 Interpretation |
33 | |||
11.15 Counterparts |
34 | |||
Exhibit A Description of SCH 619734 |
||||
Exhibit B Description of SCH 900978 |
||||
Exhibit C Existing OPKO Patent Rights |
||||
Exhibit D Technology Transfer Plan |
||||
Exhibit E Form of Press Release |
||||
Exhibit F Third Party Agreements |
||||
iii
EXCLUSIVE LICENSE AGREEMENT
This Exclusive License Agreement, made this 10th day of December, 2010 (the Effective Date),
is by and between TESARO, Inc., a Delaware company, with principal offices located at 309 Waverley
Oaks Rd., Suite 101, Waltham, MA 02452 (TESARO) and OPKO Health, Inc., a Delaware corporation,
with principal offices located at 4400 Biscayne Blvd., Miami, FL 33137 (OPKO). Each of TESARO and
OPKO may be referred to, individually, as a Party, and, collectively, as the Parties.
RECITALS
WHEREAS, OPKO owns or controls certain patent rights and know-how related to the neurokinin-l
(NK-l) receptor antagonists, SCH 619734 (Rolapitant) and SCH 900978;
WHEREAS, TESARO is interested in obtaining an exclusive license under such patent rights and
to such know-how to develop and commercialize pharmaceutical products incorporating either or both
of the foregoing compounds, and OPKO is willing to grant TESARO such a license, in each case on the
terms and conditions set forth in this Agreement.
NOW, THEREFORE, in consideration of the foregoing and the mutual covenants contained in this
Agreement, OPKO and TESARO, intending to be legally bound, hereby agree as follows:
ARTICLE I
DEFINITIONS
DEFINITIONS
When used in this Agreement, each of the following capitalized terms, whether used in the
singular or plural, shall have the meaning set forth in this Article I.
1.1. Affiliate of an entity means any person or entity which, directly or
indirectly, controls, is controlled by or is under common control with such entity. For the
purposes of this definition, control refers to any of the following: (i) direct or
indirect ownership of fifty percent (50%) or more of the voting securities entitled to vote for the
election of directors in the case of a corporation, or of fifty percent (50%) or more of the equity
interest with the power to direct management in the case of any other type of legal entity; (ii)
status as a general partner in any partnership; or (iii) any other arrangement where a person or
entity possesses, directly or indirectly, the power to direct the management or policies of an
entity, whether through ownership of voting securities, by contract or otherwise. Notwithstanding
the foregoing, the term Affiliate with respect to TESARO shall not include New Enterprise
Associates.
1.2. Agreement means this Exclusive License Agreement, including any and all
exhibits, schedules, appendices and other addenda to it and as it may be amended from time to time
in accordance with the provisions of this document.
1.3. API means the active pharmaceutical ingredient contained in Licensed Product.
1.4. API Cost means the Cost of Goods of API.
1.5. Asset Purchase Agreement means that certain Asset Purchase Agreement between
OPKO and Schering Corporation (now Merck & Co., Inc.) dated as of October 12, 2009, as amended by
letter agreement dated June 29, 2010, under which OPKO purchased certain assets related to the
Compounds.
1.6. Combination Product means any pharmaceutical product containing both a Licensed
Product component and one or more other active pharmaceutical ingredients or other significant
components.
1
1.7. Commercially Reasonable Efforts means the level of efforts and resources,
including financial resources, at least equal to those normally used by a company to conduct the
relevant activity, including, in the case of research, development or commercialization, the level
of effort and resources at least equal to those normally used by such a company to research,
develop, manufacture or commercialize, as the case may be, a product owned by such company or to
which it has rights, which product is at a similar stage in its development or product life and is
of a similar market and profitability potential to Licensed Product, taking into account all
relevant factors including the patent and other proprietary position of the product, product
labeling or anticipated labeling, market potential, financial return, medical and clinical
considerations, regulatory environment and competitive market conditions, and other technical,
legal, scientific, medical or commercial factors that such a company would deem to be relevant.
1.8. Compounds means SCH 619734 (Rolapitant) and SCH 900978.
1.9. Confidential Information means any and all information, data and materials of a
confidential or proprietary nature, which are provided by or on behalf of one Party or any of its
Affiliates to the other Party or any of its Affiliates in connection with this Agreement.
1.10. Control or Controlled, other than for purposes of Section 1.1, means the
possession of the right to grant licenses or sublicenses or to disclose proprietary or trade secret
information without violating the terms of any agreement or other arrangement with a Third Party
and without misappropriating or infringing the proprietary or trade secret information of a Third
Party.
1.11. Cost of Goods means, with respect to API or Licensed Product, as the case may
be, the aggregate of costs of TESARO or any of its Affiliates or Sublicensees to manufacture,
package, label and release such API or Licensed Product, calculated as follows: (i) to the extent
that the API or Licensed Product is manufactured, packaged, labeled or released by TESARO or any of
its Affiliates or Sublicensees, their actual direct material costs and direct labor costs plus
manufacturing overhead, directly and exclusively attributable to such API or Licensed Product
(including the API incorporated into such Licensed Product), all calculated in accordance with
GAAP; or (ii) to the extent that API or Licensed Product is manufactured, packaged, labeled or
released by a Third Party, the actual amounts paid by TESARO or any of its Affiliates or
Sublicensees to such Third Party for such activities performed on a specified quantity of such API
or Licensed Product plus the costs of any materials (including API and raw materials) provided by
TESARO or any of its Affiliates or Sublicensees to such Third Party for such activities, and any
manufacturing overhead, quality control and distribution costs incurred by TESARO or any of its
Affiliates or Sublicensees with respect to such materials provided or such Licensed Product, as
calculated in accordance with clause (i) of this Section 1.11.
1.12. Cover, Covering or Covered means, with respect to a Patent Right and
invention, that, in the absence of ownership of, or a license under, such Patent Right, the
practice of such invention would infringe a Valid Claim of such Patent Right (including in the case
of a Patent Right that is a patent application, a Valid Claim of such patent application as if such
patent application were an issued patent).
1.13. EMA means the European Medicines Agency or any successor agency.
1.14. EU means the countries of the European Union, as it is constituted as of the
Effective Date and as it may be expanded from time to time.
1.15. FDA means the United States Food and Drug Administration or any successor
agency thereto.
1.16. Field means with respect to SCH 619734 (Rolapitant) all therapeutic,
prophylactic, palliative and diagnostic uses in humans, and means, with respect to SCH 900978,
treatment of nausea or
2
vomiting of any cause; (ii) treatment of disease or treatment of symptoms or side effects of
disease in oncology indications; (iii) treatment of side effects of oncology treatments or
therapies; and (iv) any other supportive care indications in oncology.
1.17. First Commercial Sale, as to a particular country, means the first commercial
sale of a Licensed Product by TESARO or any of its Affiliates or Sublicensees to a Third Party in
such country after approval of the NDA, or if approval of an NDA is not required in such country,
then following receipt of Marketing Approval required to market such Licensed Product in such
country.
1.18. GAAP means United States generally accepted accounting principles applied on a
consistent basis, or any other accounting principles generally accepted for public companies in the
United States such as International Financial Reporting Standards (IFRS). Unless otherwise
defined or stated, financial terms shall be calculated under GAAP.
1.19. IND means an Investigational New Drug Application filed with FDA or a similar
application filed with an applicable Regulatory Authority outside of the United States such as a
clinical trial application (CTA) or a clinical trial exemption (CTX).
1.20.
Japan Income
means all royalty payments, upfront payments and development,
commercialization, regulatory approval and other milestone payments received from a Sublicensee with respect to development or commercialization of Licensed Product in Japan, less applicable Third Party Payments attributable to the development, manufacture, commercialization or use of Licensed Product in Japan, provided that, if a royalty payment made
by such Sublicensee to TESARO or any of its Affiliates includes
the purchase price of a Licensed Product, then only the royalty amount
less the Cost of Goods of such Licensed Product will be included as Japan Income.
For the sake of clarity, Japan Income will not include any of the following amounts received
by TESARO or any of its Affiliates from a Sublicensee: (i) debt financing or equity
(including conditional equity, such as warrants, convertible debt and the like) investments
in TESARO or any of its Affiliates except the portion of such financing or investment that
exceeds the fair market value of such debt or equity securities; (ii) research and development funding,
including funding for all or a portion of the costs of clinical trials or regulatory activities, which is
reimbursed to TESARO or any of its Affiliates for expenditures actually incurred by TESARO or such Affiliate
and which are directly attributable to development and commercialization of Licensed Product in Japan,
including funding provided for clinical trials and other activities conducted outside of Japan but which
will generate data to be used to support development or commercialization in Japan; (iii) reimbursement
for Cost of Goods for product manufactured by or for TESARO or any of its Affiliates; and (iv) amounts
paid by a Sublicensee to TESARO or any of its Affiliates as reimbursement for specific costs actually
incurred by TESARO or such Affiliate and which are directly attributable to development and commercialization of
Licensed Product in Japan, including reimbursement of patent-related costs for prosecution or protection of
patents in Japan, and the costs of maintaining the global safety database.
1.21. Japan Income Sharing Term has the meaning set forth in Section 4.6(b).
1.22. Know-how means all biological materials and other tangible materials,
inventions, practices, methods, protocols, formulas, knowledge, know-how, trade secrets, processes,
procedures, specifications, assays, skills, experience, techniques, data and results of
experimentation and testing, including pharmacological, toxicological, safety, stability and
pre-clinical and clinical test data and analytical and quality control data, patentable or
otherwise.
1.23. Licensed Product means any product comprising, incorporating or containing any
Compound, or an alternate form of any Compound, including, but not limited to, a pharmaceutically
acceptable salt, polymorph, crystal form, prodrug, or solvate of any Compound to the extent such
alternate form is claimed in the OPKO Patent Rights.
1.24. Major EU Markets means the United Kingdom, France, Italy, Spain and Germany.
1.25. Marketing Approval means any approval, including price approval, registration,
license or authorization from any Regulatory Authority required to market and sell a Licensed
Product in a jurisdiction and shall include an approval, registration, license or authorization
granted in connection with an NDA.
3
1.26. NDA means a New Drug Application, Biologics License Application or equivalent
submission filed with the FDA in connection with seeking Marketing Approval of a Licensed Product,
or an equivalent application filed with any equivalent regulatory agency or governmental authority
in any jurisdiction other than the United States.
1.27. Net Sales means the gross amount invoiced on sales of Licensed Product in the
Territory (not including sales of Licensed Product by a Sublicensee in Japan) by TESARO, its
Affiliates or Sublicensees to any Third Party, less the following deductions with respect to the
sale of such Licensed Product:
(i) normal trade, cash and quantity discounts and other customary discounts actually given to
customers in the ordinary course of business;
(ii) rebates, credits and allowances given by reason of rejections, returns, damaged or
defective product or recalls;
(iii) government-mandated rebates and any other compulsory payments, credits, adjustments and
rebates actually paid or deducted;
(iv) price adjustments, allowances, credits, chargeback payments, discounts, rebates, fees,
reimbursements or similar payments granted to managed care organizations, group purchasing
organizations or other buying groups, pharmacy benefit management companies, health maintenance
organizations and any other providers of health insurance coverage, health care organizations or
other health care institutions (including hospitals), health care administrators or patient
assistance or other similar programs, or to federal, state/provincial, local and other governments,
including their agencies, or to wholesalers, distributors or other trade customers;
(v) reasonable and customary freight, shipping, insurance and other transportation expenses,
if actually borne by such TESARO or its Affiliates or Sublicensees without reimbursement from any
Third Party;
(vi) sales, value-added, excise taxes, tariffs and duties, and other taxes and government
charges directly related to the sale, delivery or use of Licensed Product (but not including taxes
assessed directly against the income derived from such sale) net of any credits or allowances
received by TESARO or its Affiliates or Sublicensees with respect to such taxes or charges;
(vii) amounts previously included in Net Sales of Licensed Product that are written off as
uncollectible after reasonable collection efforts, in accordance with standard practices of the
applicable party; and
(viii) any item, substantially similar in character or substance to any of the foregoing,
calculated in accordance with GAAP consistently applied and customary in the pharmaceutical
industry to be deducted in the definition of net sales in a license agreement of this type.
Notwithstanding anything in this Agreement to the contrary, the transfer of a Licensed Product
between or among TESARO, its Affiliates and Sublicensees will not be considered a sale.
Net Sales will include the cash consideration received on a sale and the fair market value of all
non-cash consideration.
Disposition of Licensed Product for, or use of the Licensed Product in, clinical trials or other
scientific testing, as free samples, or under compassionate use, patient assistance, or test
marketing programs or other similar programs or studies where a Licensed Product is supplied
without charge shall not result in
4
any Net Sales however if TESARO or any of its Affiliates or Sublicensees charges for such Licensed
Product, the amount billed will be included in the calculation of Net Sales.
Net Sales will be determined from books and records maintained in accordance with GAAP,
consistently applied throughout the organization and across all products of the entity whose sales
of Licensed Product are giving rise to Net Sales.
In the event a Licensed Product is sold in the form of a Combination Product, then the Net Sales
for any such Combination Product shall be determined by multiplying the Net Sales of the
Combination Product during the applicable royalty reporting period, by the fraction, A/(A+B), where
A is the weighted (by sales volume) average sale price of the Licensed Product component when sold
separately in finished form in the country in which the Combination Product is sold and B is the
weighted (by sales volume) average sale price of the other active pharmaceutical ingredients or
significant components included in the Combination Product when sold separately in finished form in
the country in which the Combination Product is sold, in each case during the applicable royalty
reporting period or, if sales of both the Licensed Product component and the other active
pharmaceutical ingredients or significant components did not occur in such period, then in the most
recent royalty reporting period during the preceding twelve (12) months in which sales of both
occurred, if any. In the event that such average sale price cannot be determined for both the
Licensed Product and all other active pharmaceutical ingredients or significant components included
in the Combination Product, then the Parties will in good faith discuss and agree on a pro-rata
allocation of the Net Sales that reflects the Licensed Products contribution to the Combination
Product on an equitable basis. TESARO covenants that neither it nor any of its Affiliates or
Sublicensees will intentionally manipulate the fraction A/(A+B) to avoid or reduce royalty payments
or obligations that would otherwise be due for sales of Licensed Product in combination form or
otherwise
1.28. OPKO Patent Rights means (i) any and all patents and patent applications owned
or otherwise Controlled by OPKO or any of its Affiliates on the Effective Date or at any time
during the Term anywhere in the Territory that Cover OPKO Know-how or that otherwise Cover the
research, formulation, development, manufacture, import, marketing, sale or use of Licensed Product
in the Field; and (ii) any and all extensions or restorations of the foregoing patents or patent
applications by existing or future extension or restoration mechanisms, including revalidations,
reissues, re-examinations and supplementary protection certificates and the like. OPKO Patent
Rights includes the patents and patent applications listed in Exhibit C.
1.29. OPKO Japan Share has the meaning set forth in Section 4.5, subject to
adjustment as set forth in Section 4.7.
1.30. OPKO Know-how means any Know-how owned or otherwise Controlled by OPKO or any
of its Affiliates as of the Effective Date or any time during the Term that (i) is incorporated
into Licensed Product or the manufacturing process for Licensed Product; (ii) was used or generated
in the development, manufacture or use of Licensed Product; or (iii) is otherwise reasonably
necessary or useful to the research, formulation, development (including filing for and obtaining
Marketing Approval), manufacture, import, marketing, sale or use of Licensed Product in the Field.
1.31. Patent Rights means patents and patent applications and all substitutions,
divisions, continuations, continuations-in-part, reissues, reexaminations, supplemental protection
certificates and extensions and the like thereof, and all counterparts thereof in any country.
1.32. Regulatory Authority means any federal, national, multinational, state,
county, city, provincial, or local regulatory agency, department, bureau or other governmental
entity with authority over the marketing, commercialization, manufacture or sale of a
pharmaceutical product in the Territory, including the FDA in the United States and the EMA in the
EU.
5
1.33. Royalty Term has the meaning set forth in Section 4.6 (a).
1.34. SCH 619734 (Rolapitant) means the compound described in Exhibit A.
1.35. SCH 900978 means the compound described in Exhibit B.
1.36. Sublicensee means a Third Party to whom TESARO or any of its Affiliates or
another Sublicensee grants an express sublicense under the OPKO Patent Rights and OPKO Know-how to
develop, manufacture, commercialize or use Licensed Product in the Field, provided that the term
Sublicensee does not include any wholesaler or third party distributor who re-sells a Licensed
Product purchased from TESARO or any of its Affiliates or Sublicensees in final finished form (but
not necessarily in final packaged, and labeled form), provided that OPKO is paid the royalty
specified in Section 4.4 on the purchase price of such Licensed Product paid by such wholesaler or
distributor to TESARO or any of its Affiliates or Sublicensees.
1.37. Technology Transfer Plan means the plan for transfer to TESARO of OPKO
Know-how attached to this Agreement as Exhibit D.
1.38. Term means the term of this Agreement determined in accordance with Section
9.1.
1.39. Territory means worldwide.
1.40. TESARO Improvement means any Know-how owned or otherwise Controlled by
TESARO or any of its Affiliates that constitutes an improvement of the OPKO Know-how
developed during the Term and is incorporated into the Licensed Product by TESARO or any of
its Affiliates.
1.41. TESARO Improvement Patent Rights means Patent Rights owned or Controlled by
TESARO or any of its Affiliates Covering any TESARO Improvement.
1.42. Third Party means any person other than a Party or any of its Affiliates or
their respective employees.
1.43. Third Party Agreements has the meaning set forth in Section 7.2(k).
1.44. Third Party Payments means all **** under licenses to intellectual property
or to acquire intellectual property that is necessary for the development, manufacture,
import, sale or use of Licensed Product in the Field. For purposes of this definition, the
term necessary shall mean that, in the reasonable determination ****, if the relevant
Patent Right of the Third Party were to be found to be valid, there would be **** that the
manufacture, use or sale of Licensed Product would be found to infringe such Patent Right,
provided that nothing in the foregoing requires a court or other legal determination of
validity or infringement.
1.45. United States or U.S. means the United States of America and its
territories and possessions.
1.46. Valid Claim means (i) a claim of an issued and unexpired patent that has not
been revoked or held unenforceable or invalid by a decision of a court or other governmental
agency of competent jurisdiction from which no appeal can be taken or with respect to which
an appeal is not taken within the time allowed for appeal, and that has not been disclaimed
or admitted to be invalid or unenforceable through reissue, disclaimer or otherwise or been
dedicated to the public, and (ii) a claim in a pending patent application that is being
prosecuted and that has not been abandoned, disclaimed, allowed to lapse or finally
determined to be unallowable by the
6
applicable governmental authority in a decision from which no appeal can be taken or from
which no appeal is taken within the time allowed for appeal.
ARTICLE II
LICENSE GRANT
LICENSE GRANT
2.1. License Grant. Subject to the terms and conditions of this Agreement, OPKO and
its Affiliates grant to TESARO an exclusive license (or sublicense, as the case may be) under the
OPKO Patent Rights and the OPKO Know-how, in each case with the right to grant sublicenses, to the
extent provided in Section 2.2, to research, develop, make, have made, use, import, export, market,
offer for sale, sell and have sold, Licensed Product in the Territory within the applicable Field.
2.2. Sublicenses.
(a) Sublicensing. The rights granted to TESARO by OPKO under Section 2.1, may be
extended to an Affiliate or sublicensed, in whole or in part, to a Third Party (through multiple
levels of sublicensing); provided, that any sublicense that includes commercialization rights will
require the prior consent of OPKO which such consent OPKO shall not unreasonably withhold,
condition or delay. Notwithstanding anything in this Agreement to the contrary, OPKO shall be
deemed to have granted its consent to any sublicense under this Section if OPKO has not provided
TESARO with written notice of OPKOs reasonable objection to the sublicense within **** business
days of receipt of a written request for such consent from TESARO, along with an unredacted copy of
the relevant term sheet for an agreement which transfers rights granted hereunder so that OPKO may
consider granting consent. In addition, TESARO will, promptly after signature, provide OPKO with an
unredacted copy of each agreement with a Sublicensee executed by TESARO or any of its Affiliates.
Permitted Sublicensees may also extend the rights granted under Section 2.1 to any of their
Affiliates.
(b) Performance by Sublicensees. TESARO will be fully responsible for performance by
each Sublicensee of its obligations under this Agreement. Each sublicense granted by TESARO
pursuant to this Section 2.2 will contain terms and conditions consistent with those sections of
this Agreement applicable to Sublicensees. Each sublicense agreement will contain the following
provisions: (i) a requirement that any Sublicensee selling Licensed Product submit applicable sales
or other reports to TESARO to the extent necessary or relevant to the reports required to be made
or records required to be maintained under this Agreement; (ii) an audit requirement as to those
Sublicensees selling Licensed Product consistent with that set forth in Section 4.15; and (iii) a
requirement that such Sublicensee comply with the confidentiality provisions and restrictions on
use of Confidential Information consistent with Article VI with respect to Confidential Information
of OPKO. If TESARO becomes aware of a material breach by a Sublicensee of the rights granted to
TESARO under Section 2.1, TESARO will promptly notify OPKO of the particulars of the same, and will
use Commercially Reasonable Efforts to enforce the terms of such sublicense.
2.3. Covenant Not to Sue. At the request of TESARO, OPKO will use Commercially
Reasonable Efforts to enforce the covenant not to sue obligations of Merck & Co., Ltd. under
Section 7.7 of the Asset Purchase Agreement with respect to the activities of TESARO and its
Affiliates and Sublicensees under this Agreement; provided, that TESARO will promptly reimburse all
out-of-pocket expenses (including reasonable attorneys fees and expenses) incurred by OPKO in
connection with such requested efforts.
2.4. Responsibility; Decision-making. During the Term, TESARO will, including through
its Affiliates and Sublicensees, have sole responsibility for and sole decision-making authority
with respect to, the research, development, manufacture, marketing, sale and use of Licensed
Product in the Field, and except as otherwise expressly set forth in this Agreement, will be
responsible for all of the costs and expenses associated with such activities during the Term.
7
2.5. Diligence. TESARO will use Commercially Reasonable Efforts during the Term to
develop and obtain Marketing Approval for a Licensed Product in the United States and in each Major
EU Market, and to commercialize such Licensed Product in the United States and each Major EU Market
if the relevant Marketing Approval is obtained. TESARO shall keep OPKO informed as to TESAROs
progress in these efforts. In addition, TESARO will use Commercially Reasonable Efforts to secure
any data and market exclusivity, including New Chemical Entity exclusivity, for a Licensed Product
for which Marketing Approval is obtained to the extent available from the applicable Regulatory
Authorities. TESARO agrees to register this Agreement with any foreign governmental agency, which
requires such registration and where the failure to so register would have a material adverse
impact on commercialization of Licensed Product in a major market, and **** in connection
therewith. TESARO shall not be relieved of any of its obligations under this Agreement by any
failure to register this Agreement in any country, and, specifically, shall not be relieved of its
obligation to make any payment due to OPKO where such payment is blocked due to any failure to
register this Agreement.
2.6. Joint Steering Committee.
(a) Formation. Within **** days after the Effective Date, the parties will form a
committee (the Joint Steering Committee) comprising at least **** representatives from each
party.
(b) Responsibilities. The Joint Steering Committee will be responsible for (i)
reviewing the status and progress of efforts related to the development, manufacture and
registration of the Licensed Product; and (ii) discussing other matters related to this Agreement
referred to it by agreement of the Parties. Each Partys representatives to the Joint Steering
Committee shall communicate with one another as necessary to perform the Parties respective
obligations under this Agreement.
(c) Meetings. The Joint Steering Committee shall hold its first meeting in person
within forty-five (45) days after the Effective Date. Thereafter, the Joint Steering Committee will
meet as often as necessary either in person or by telephone at mutually acceptable times and
locations. Either party may call a Joint Steering Committee meeting upon reasonable written notice,
but not more than twice each year, unless both Parties mutually agree.
(d)
Development Plan. The parties agree that TESARO shall prepare a written development
plan (the Development Plan) for the development of the Licensed Product within forty-five (45)
days after the Effective Date. The Development Plan shall include schedules and milestones for the
development activities of TESARO. TESARO may amend the Development
Plan at any time in its sole discretion. TESARO shall
present the Development Plan and any material amendments to the Joint Steering Committee for
review.
2.7. Latin America. OPKO will have the option to become the exclusive distributor of
Licensed Product in Latin America on terms to be mutually agreed upon by the Parties (the
Latin America Option. To exercise its Latin America Option, OPKO must give written notice
of such exercise to TESARO within **** after **** for Licensed Product in the **** . In the event
OPKO does not give notice of its exercise of the Latin America Option within the foregoing time
period or the Parties are unable, despite good faith negotiation, to agree on mutually acceptable
terms of a distribution agreement, OPKO will have no further rights under this Section, and TESARO
will be free to distribute Licensed Product in Latin America itself or through an Affiliate,
Sublicensee or a Third Party distributor. Notwithstanding the foregoing, in the event the Parties
are unable, despite good faith negotiation, to agree on mutually acceptable terms of a distribution
Agreement, TESARO agrees that it will not enter into a final agreement with any Third Party
regarding the rights to distribute Licensed Product in all of or any territory within Latin America
(a Latin American Opportunity) without first giving OPKO a good faith opportunity to
agree to such Latin American Opportunity on material terms substantially similar to those offered
(or intended to be offered) by TESARO to the Third Party (or offered by the Third Party to
8
TESARO) (Right to Match). OPKOs Right to Match with regard to Latin American
Opportunities operates as follows:
(a) When a Latin American Opportunity arises, TESARO shall give OPKO prompt written notice of
the material financial, intellectual property, term and termination, indemnification, governing law
and other material terms of the Latin American Opportunity. Within **** after receiving TESAROs
written notice under this Subsection 2.7(a), OPKO shall respond in writing to TESARO regarding
whether it will substantially match or decline to substantially match the material terms of
TESAROs proposed agreement.
(b) If, in its response, OPKO indicates its interest in substantially matching the material
terms of TESAROs proposed agreement, the Parties shall negotiate in good faith a definitive
agreement (with material terms substantially similar to those set forth in TESAROs proposed
agreement) for a period of up to **** after TESARO received OPKOs response. If, after such time, a
final agreement cannot be reached and the Parties do not mutually extend the negotiation period,
TESARO shall be free to execute its proposed agreement with the Third Party on material terms no
more favorable to the Third Party than the material terms presented to OPKO under subsection (a)
above were to OPKO. However, if such material terms are more favorable to the Third Party, then
TESARO must offer, and OPKO has a Right to Match, such terms in accordance with the procedures and
restrictions contained in this Section 2.7.
ARTICLE III
TECHNOLOGY TRANSFER AND TRANSITION ACTIVITIES
TECHNOLOGY TRANSFER AND TRANSITION ACTIVITIES
3.1.
Know-how Transfer. OPKO agrees to transfer to TESARO the OPKO Know-how specified
in the Technology Transfer Plan in accordance with the time-lines and other requirements set forth
in such plan, and to transfer such other OPKO Know-how, as TESARO may from time to time reasonably
request during the Term, promptly after such request. In addition, OPKO will, as part of transfer
of OPKO Know-how, assign to TESARO those Third Party Agreements as to which TESARO specifically
requests assignment and which by their terms may be assigned. To the extent the consent of any
Third Party is required to assign a Third Party Agreement to TESARO, OPKO will use Commercially
Reasonable Efforts to obtain such consent. In the event a Third Party Agreement is not assigned to
TESARO, OPKO will, as set forth in the Technology Transfer Plan, or as otherwise requested by
TESARO, use Commercially Reasonable Efforts to obtain any information or other benefits under such
agreement related to access to OPKO Know-how as would be available to OPKO.
3.2. Cooperation. OPKO shall make its personnel reasonably available to TESARO to
respond to questions related to the OPKO Know-how, and to provide such ongoing support and
assistance as TESARO may reasonably request in the transition of development and manufacturing
responsibility for Licensed Products to TESARO. In connection with the foregoing, at the request of
TESARO, OPKO will seek the assistance of Merck & Co., Ltd. to the extent such support continues to
be available under Section 2.5 of the Asset Purchase Agreement.
3.3. Regulatory Transition. Within **** after the Effective Date, or as otherwise
mutually agreed, the Parties will file with applicable Regulatory Authorities such documentation as
may be required to transfer any IND to TESARO, and the Parties will use Commercially Reasonable
Efforts to take such actions as any such Regulatory Authority may request to effect any such
transfer. Prior to transfer of the IND, OPKO will continue to perform such obligations as are
required under applicable law with respect to an IND holder, but under the direction of TESARO.
3.4.
Supply of Material. OPKO will, **** transfer to TESARO in accordance with the Technology
Transfer Plan all quantities of API and Licensed Product in OPKOs possession or control. To the
extent such API or Licensed Product is identified as GMP-grade materials in the Technology Transfer
Plan,
9
OPKO represents that (i) since OPKOs acquisition of such materials, OPKO has handled and
stored such materials in accordance with current Good Manufacturing Practices as defined in the
U.S. (GMP), and (ii) nothing has come to OPKOs attention which leads it to believe that
any such material has not been manufactured and stored in accordance with GMP, that it would not
conform in all material respects to the applicable specifications or would not be fit for use in
clinical trials pursuant to FDA guidelines and requirements. OPKO will provide copies of batch
records and certificates of compliance in its possession with respect to such material. In
addition, OPKO will, at the request of TESARO, require Merck & Co., Inc. to deliver the Hold Back
API, as defined in the Asset Purchase Agreement, to TESARO or its designee, and to supply addition
quantities of API to the extent consistent with Merck & Co., Inc.s obligation under Section
7.11(b) of the Asset Purchase Agreement on terms to be approved by TESARO.
3.5. Costs. Each Party **** associated with technology transfer activities to be
provided under this Section. To the extent any technology transfer activities to be provided under
this Section require **** shall bear the costs of such external resources, provided that such activities
and costs are expressly set forth in the Technology Transfer Plan or are otherwise approved in
writing in advance by ****.
ARTICLE IV
FINANCIAL PROVISIONS
FINANCIAL PROVISIONS
4.1. License Fee. Within ten (10) days of the Effective Date, TESARO will pay to OPKO a
non-creditable, non-refundable license fee of $6,000,000, as compensation for past and future
research and development expenses, patent prosecution and maintenance fees, and for exclusive
rights to the Licensed Product in the U.S.
4.2. Intentionally Left Blank
4.3. Milestones Payments by TESARO. Subject to the terms and conditions of this
Agreement, TESARO will pay OPKO a milestone payment upon the first occurrence of each of the
following events, no later than thirty (30) days after the occurrence of the event:
| Event Milestone | Event Milestone Payment | |||
(i) Acceptance by the FDA of the first NDA for
Marketing Approval of Licensed Product in the
United States |
$ | 5,000,000 | ||
(ii) First Commercial Sale of Licensed Product in
the U.S. |
$ | 15,000,000 | ||
(iii) First Commercial Sale of Licensed Product in
the EU |
$ | 10,000,000 | ||
Each of the above milestone payments will be payable only upon the first occurrence of the
applicable event, regardless of how many times the event is ultimately achieved.
In addition, TESARO will pay to OPKO the following commercial milestone payments upon the first
achievement of the corresponding event:
10
First achievement of calendar year Net Sales in excess of
$150 million |
$ | ****,000,000 | ||
First achievement of calendar year Net Sales in excess of
$300 million |
$ | ****,000,000 | ||
First achievement of calendar year Net Sales in excess of
$500 million |
$ | ****,000,000 |
4.4. Royalty Payments by TESARO. Subject to the adjustment, if any, to be made under
Sections 4.7 and 4.8, TESARO will pay to OPKO royalties on Net Sales of Licensed Product in the
Field in the Territory (other than sales of Licensed Product by a Sublicensee in Japan) by TESARO
and its Affiliates and Sublicensees, calculated using the following royalty rates:
(a) U.S. and EU. For the sale of Licensed Product in the U.S. and the EU, the royalty rate
will be the Tier One Royalty Rate or the Tier Two Royalty Rate, as set forth below, depending on
the applicable API Costs. The Tier One royalty rates will apply if the average API Costs of Licensed Product
sold by TESARO and its Affiliates and Sublicensees during the preceding calendar year was equal to
or greater than ****. The Tier Two royalty rates will apply if the average API Costs of Licensed Product
sold by TESARO and its Affiliates and Sublicensees during the preceding calendar year was less than
****. Notwithstanding the foregoing, the API Costs used to determine whether to apply the Tier One
Royalty Rate or the Tier Two Royalty Rate in the launch year will be based the average API Costs for the
clinical and commercial runs of API during the twelve (12) months preceding the date of First Commercial Sale.
| Portion of Calendar Year Net Sales | Tier One Royalty | Tier Two Royalty | ||||||
| in the United States | Rates | Rates | ||||||
On that portion of calendar year
Net Sales in the U. S. less than or
equal to $150 million |
****% | ****% | ||||||
On that portion of calendar year
Net Sales in the U. S. greater than
$150 million but less than or
equal to $300 million |
****% | ****% | ||||||
On that portion of calendar year Net |
****% | ****% | ||||||
Sales in the U.S. greater than
$300 million but less than or
equal to $450 million |
||||||||
On that portion of calendar year
Net Sales in the U.S. greater than
$450 million |
****% | ****% | ||||||
11
| Tier One | Tier Two | |||||||
| Portion of Calendar Year Net Sales in the | Royalty | Royalty | ||||||
| EU | Rate | Rate | ||||||
On that portion of
calendar year Net
Sales in the EU less
than or equal to
$50 million |
****% | ****% | ||||||
On that portion of
calendar year Net
Sales in the EU
greater than $50
million but less than
or equal to $100
million |
****% | ****% | ||||||
On that portion of
calendar year Net
Sales in the EU
greater than $100
million but less than
or equal to $150
million |
****% | ****% | ||||||
On that portion of
calendar year Net
Sales in the EU
greater than $150
million |
****% | ****% | ||||||
(b) Rest of World Other than Japan. The royalty rate outside the U.S., EU and Japan
will be ****.
(c) Minimum Annual Royalty.
If the aggregate amount of royalties paid or payable by TESARO to OPKO on sales of Licensed Products under this Section during each of
the first five calendar years commencing with the first full calendar year following the First Commercial Sale of Licensed Product in the
U.S. and ending with the calendar year in which the fifth anniversary of the First Commercial Sale of Licensed Product in the U.S. occurs
(the Measurement Period) is less than **** (the
Minimum Annual Royalty), then within forty-five (45) days of the end of each such
calendar year, TESARO will pay OPKO an amount equal to the difference between the **** and the aggregate amount of royalties paid
or payable to OPKO on sales of the Licensed Product during such
calendar year (the Annual Royalty Shortfall). During the Measurement
Period, any Annual Royalty Shortfall paid or payable by TESARO will be offset dollar for dollar by the aggregate amount of royalties
paid or payable to OPKO on sales of the Licensed Product during any prior calendar year during the Measurement Period that exceeded
**** and any royalties payable during the Measurement Period that exceed **** will be offset by the amount of any Annual Royalty
Shortfall payments made in any prior calendar year and not previously used as an offset. The Minimum Annual Royalty will not apply,
and no Annual Royalty Shortfall will be due, in the event the commercial potential of any Licensed Product in the U.S. has been
materially adversely affected by: (i) the outcome of a clinical trial of Licensed Product; (ii) material safety issues identified in the course
of the Phase 3 clinical trial or commercialization of Licensed Product; (iii) restrictions imposed by third party payers, including the
government, on reimbursement for Licensed Product; or (iv) the absence of a Valid Claim of an issued patent within OPKO Patent Rights
Covering the Licensed Product in the United States.
4.5. Japan.
TESARO will pay OPKO fifty percent (50%) of all Japan Income (the
OPKO Japan Share). In the event OPKO is required
to make payments to a Third Party under an agreement in existence as of the Effective Date, based on the OPKO Japan Share of the
Japan Income (the OPKO Third Party Obligation). TESARO will pay up to fifty percent (50%) of such OPKO Third Party Obligation,
provided that, in no event will TESAROs share of the OPKO Third Party Obligation exceed twelve and one half percent (12.5%) of the
amounts that would otherwise be payable to OPKO on the Japan Income that triggered the OPKO Third Party Obligation. In the event
sales of Licensed Product in Japan are made directly by TESARO or any of its Affiliates and are not conducted through a Sublicensee,
TESARO will pay to OPKO royalties on Net Sales of Licensed Product in
Japan at the rate of ****, subject to the adjustments set forth
in Section 4.7 and 4.8 to the same extent as applicable to royalties on Net Sales payable under Section 4.4.
4.6. Royalty and Income Sharing Term.
(a) Royalties. Royalties under Section 4.4 will be payable on a country by country and
Licensed Product-by-Licensed Product basis during the period commencing on the First Commercial
Sale of such Licensed Product in the applicable Field in such country and ending upon the later of
(i) the date of expiration, unenforceability or invalidation of the last Valid Claim of OPKO Patent
Rights
12
Covering
such Licensed Product in such country, and (ii) twelve (12) years from the date of First
Commercial Sale in such country (the Royalty Term).
(b) Japan Income Sharing. TESAROs obligation to share Japan Income under Section
4.5 will be payable on a Licensed Product-by-Licensed Product basis during the period commencing on
the Effective Date and ending upon the later of (i) the date of expiration, unenforceability or
invalidation of the last Valid Claim of OPKO Patent Rights Covering Licensed Product in Japan, and
(ii) twelve (12) from the date of First Commercial Sale in Japan (Japan Income Sharing Term).
(c) End of Royalty Term or Japan Income Sharing Term. Upon expiration of the
Royalty Term or Japan Income Sharing Term, as the case may be, in the country of sale, the license
granted to TESARO and its Affiliates and Sublicenses under Article II will convert to a fully
paid-up, non-royalty-bearing, license in the applicable country.
4.7. Reduction for No Valid Claim. The royalties payable under Section 4.4 with
respect to Net Sales of a Licensed Product will be reduced, on a country by country and Licensed
Product-by-Licensed Product basis, by **** of the amounts otherwise payable under Section 4.4,
during any portion of the Royalty Term when there is no Valid Claim of an issued patent within OPKO
Patent Rights Covering such Licensed Product in the country of sale or other protective data or
marketing exclusivity. Notwithstanding the foregoing, in the event there is no Valid Claim of an
issued patent within OPKO Patent Right Covering a Licensed Product being sold in a country and a
Third Party has obtained Marketing Approval in such country for a product containing the same
active ingredient as contained in Licensed Product, the reduction on royalties under the preceding
sentence will be increased to ****.
4.8. Third Party Payments.
(a) OPKO
Payments. Except as specifically set forth in Section 4.5,
OPKO will pay
all milestones and other payments due under the Asset Purchase
Agreement, and under any other agreement to which OPKO or
any of its Affiliates is a party.
(b) Other
Third Party Payments. TESARO will have the right to deduct from
royalties otherwise
payable to OPKO under Section 4.4 (after application of the deductions set forth in Section 4.7),
fifty percent (50%) of Third Party Payments, provided that in no event will the royalty payable to OPKO on Net
Sales of Licensed Product be reduced as a result of application of this paragraph, to less than
fifty percent (50%) of the amount otherwise payable under Section 4.4, as reduced by Section 4.7. Amounts
available for offset under this Section and not used as a credit against royalties in the period
incurred may be carried over to future periods until fully utilized.
4.9. Payments; Reports. TESARO will pay royalties due on Net Sales and amounts due
with respect to Japan Income received in a calendar quarter within **** days of the end of such
calendar quarter. Within **** days after the end of each calendar quarter for which amounts are
payable by TESARO under Section 4.4 or 4.5, TESARO will submit to OPKO a report, on a
country-by-country basis, providing in reasonable detail an accounting of all Net Sales by TESARO
and its Affiliates and Sublicensees in the Territory (including, in each case, an accounting of all
unit sales of the Licensed Product and a calculation of the deductions from gross invoice price to
Net Sales in accordance with Section 1.27) made during such calendar quarter and all Japan Income
and the calculation of the applicable amounts due under Section 4.4 and 4.5. TESARO will, at the
time TESARO submits a report under this Section, pay to OPKO all amounts due to OPKO under Sections
4.4 and 4.5, as indicated in the applicable report.
4.10. Taxes. TESARO will make all payments to OPKO under this Agreement without
deduction or withholding except to the extent that any such deduction or withholding is required by
applicable law to be made on account of Taxes (as that term is defined below). Any Tax required to
be
13
withheld under applicable law on amounts payable under this Agreement will promptly be paid by
TESARO or its Affiliates or Sublicensees on behalf of OPKO to the appropriate governmental
authority, and TESARO will furnish OPKO with proof of payment of such Tax. Any such Tax required to
be withheld will be an expense of and borne by OPKO. TESARO will give notice of its intention to
begin withholding any such Tax in advance and cooperate to use reasonable and legal efforts to
reduce such Tax on payments made to OPKO hereunder. The Parties will cooperate with respect to all
documentation required by any relevant government taxing authority or reasonably requested by
either Party to secure a reduction in the rate of applicable withholding Taxes. Solely for purposes
of this Section 4.10, Tax or Taxes means any present or future taxes, levies, imposts, duties,
charges, assessments or fees of any nature (including interest, penalties and additions thereto)
that are imposed by a government authority, but not including TESARO income taxes.
4.11. United States Dollars. All dollar ($) amounts specified in this Agreement
are United States dollar amounts.
4.12. Currency Conversion. All payments to be made by TESARO to OPKO will be made
in U.S. Dollars, to a bank account designated by OPKO. In the case of sales outside the United
States, payments received by TESARO will be expressed in the U.S. Dollar equivalent calculated on a
quarterly basis in the currency of the country of sale and converted to their U.S. Dollar
equivalent using the average rate of exchange over the applicable calendar quarter to which the
sales relate, in accordance with GAAP and the then current standard methods of TESARO or the
applicable Sublicensee, to the extent reasonable and consistently applied. TESARO will inform OPKO
as to the specific exchange rate translation methodology used for a particular country or
countries.
4.13. Blocked Payments. If, by reason of applicable laws or regulations in any
country, it becomes impossible or illegal for TESARO or any of its Affiliates or Sublicensees to
move revenues related to Licensed Product out of such country, TESARO will promptly notify OPKO of
the conditions preventing such transfer, and royalties on the affected Net Sales or amounts payable
on Japan Income shall, in lieu of payment under Section 4.9, be deposited in local currency in the
relevant country to the credit of OPKO in a recognized banking institution in such county
designated by OPKO or, if none is designated by OPKO within a period of thirty (30) days, in a
recognized banking institution in such county selected by TESARO or its Affiliates or Sublicensees,
as the case may be, and identified in a notice given to the Party on whose account the funds are
deposited.
4.14. Late Payments. TESARO will pay interest to OPKO on the aggregate amount of
any payments that are not paid on or before the date such payments are due under this Agreement at
a rate per annum equal to the lesser of **** per month or the highest rate permitted by
applicable law, calculated based on the number of days such payments are paid after the date such
payments are due.
4.15. Records and Audits. TESARO will keep complete and accurate records relating
to the calculations of Net Sales and Japan Income generated in the then current calendar year, and
during the preceding ****. OPKO will have the right, **** at its ****, to have a nationally
recognized, independent, certified public accounting firm, selected by it and reasonably acceptable
to TESARO, review any such records of TESARO and its Affiliates and Sublicensees (the Audited
Party) in the location(s) where such records are maintained by the Audited Party upon
reasonable written notice (which shall be no less than thirty (30) days prior written notice) and
during regular business hours and under obligations of strict confidence, for the sole purpose of
verifying the basis and accuracy of payments made under Section 4.4 and 4.5 within the **** period
preceding the date of the request for review. No **** will be subject to audit under this Section
more than once. TESARO will receive a copy of each such report concurrently with receipt by OPKO.
Should such inspection lead to the discovery of a discrepancy to OPKOs detriment, TESARO will,
within thirty (30) days after receipt of such report from the accounting firm, pay any undisputed
amount of the discrepancy, plus interest on the underpayment at a rate per annum equal to the
lesser of **** per month or the highest rate permitted by applicable law,
14
calculated from the date the underpayment was made until the date of payment to OPKO of the
underpayment. **** will pay the full cost of the review unless the underpayment of amounts due to
**** is greater than **** of the amount due for the entire period being examined, in which case
**** will pay the reasonable cost charged by such accounting firm for such review. Any undisputed
overpayment of royalties by TESARO revealed by an examination will be paid by OPKO within **** of
OPKOs receipt of the applicable report. Any disagreement regarding the results of any audit
conducted under this Section will be subject to the dispute resolution provisions set forth in
Article X.
ARTICLE V
INTELLECTUAL PROPERTY OWNERSHIP, PROTECTION
AND RELATED MATTERS
INTELLECTUAL PROPERTY OWNERSHIP, PROTECTION
AND RELATED MATTERS
5.1. Prosecution and Maintenance of Patent Rights. Within **** after the Effective
Date, OPKO will transfer to TESARO responsibility for filing, prosecuting and maintaining all OPKO
Patent Rights (other than the OPKO Patent Rights, if any, that were licensed but not assigned to
OPKO under the Asset Purchase Agreement) in such a way that there is not any loss of rights during
such **** period or in connection with the transition, including consulting with TESARO and
cooperating with TESARO related to such activities prior to completion of the transition, and
contacting the foreign agents of OPKO to assist in the transfer of power of attorney as required by
the relevant patent offices for TESARO to assume prosecution of such files. Commencing after
notification to the USPTO and OPKO foreign agent of the change in prosecution status, TESARO will
have responsibility, at TESAROs cost, for filing, conducting prosecution, and maintaining
(including the defense of any interference or opposition proceedings) all such OPKO Patent Rights
as to which OPKO has assumed and maintains responsibility under this Section, and shall use
Commercially Reasonable Efforts in the conduct of such activities. TESARO will provide to OPKO
copies of all prosecution filings and material submissions and correspondence related to OPKO
Patent Rights for which TESARO has assumed and maintains responsibility under this Section sent to
or received from patent offices, and other service providers including maintenance fee providers,
and, with respect to patent applications, and material submissions, will use reasonable efforts to
provide OPKO with a draft of each such filing or material submission reasonably in advance of
submission, and will consider in good faith any comments that OPKO may timely provide. In addition,
TESARO will provide to OPKO such other information related to prosecution of the OPKO Patent Rights
for which TESARO has assumed and maintains responsibility under this Section as OPKO may from time
to time reasonably request to allow OPKO to track prosecution and maintenance of such OPKO Patent
Rights including docket reports of all pending and issued patents and patent applications within
OPKO Patent Rights. In the event TESARO decides to abandon prosecution in any country with respect
to an OPKO Patent Right for which TESARO is responsible under this Section in a particular country
or decides to not otherwise maintain or extend any OPKO Patent Right for which TESARO is
responsible under this Agreement in a particular country, in either case where a substitute is not
filed for such OPKO Patent Right (such OPKO Patent Right in the applicable country being referred
to in this Agreement as an Abandoned Patent Right), TESARO will give OPKO written notice, and
will transfer the relevant files and authority to OPKO, sufficiently in advance of any loss of
rights to allow OPKO to file, prosecute, maintain or extend, as the case may be, claims with
respect to such Abandoned Patent Rights in the relevant country, and such Abandoned Patent Right in
the relevant country will no longer be included as an OPKO Patent Right licensed to TESARO under
Agreement.
5.2. Patent Term Extensions. TESARO will use Commercially Reasonable Efforts to
obtain patent term extensions (including those extensions available under U.S. Drug Price
Competition and Patent Term Restoration Act of 1984, the Supplementary Certificate of Protection of
Member States of the EU and other similar measures in any other country) wherever applicable to
licensed OPKO Patent Rights as to which TESARO controls prosecution that Cover Licensed Product in
the Field in the Territory, and OPKO will cooperate, at TESAROs request and expense in connection
with such activities. All filings for such extensions shall be made by the Party responsible for
filing, prosecuting and maintaining the relevant Patent Rights in accordance with this Section.
15
5.3. Third Party Infringement.
(a) Notices. Each Party will promptly report in writing to the other Party any (i)
known or suspected infringement of any OPKO Patent Rights, or (ii) unauthorized use or
misappropriation of any OPKO Know-how by a Third Party, of which such Party becomes aware, in each
case only to the extent relevant to Licensed Product or the development, manufacture,
commercialization or use of Licensed Product in the Field in the Territory, and will provide the
other Party with all available information evidencing such infringement, or unauthorized use or
misappropriation.
(b) TESARO First Right to Enforce Certain OPKO Patent Rights. TESARO or its
designated Affiliate or Sublicensee will have the first right, but not the obligation, to initiate
a suit or take other appropriate action that it believes is reasonably required to prevent or abate
actual or threatened infringement or misappropriation of, or otherwise protect or enforce, the OPKO
Patent Rights as to which TESARO controls prosecution against a Third Party who is researching,
developing, making, using or selling a product in the Field in a country within the Territory. OPKO
and its Affiliates will join such suit if the relevant court would lack jurisdiction if OPKO or
such Affiliate were absent from such suit and OPKO and such Affiliates will execute such legal
papers and cooperate in the prosecution of such suit as may be reasonably requested by TESARO;
provided, that **** incurred by **** and such Affiliates in connection with such requested
cooperation.
(c) OPKO Rights if TESARO Elects Not to Proceed. If TESARO does not initiate a
suit or take other appropriate action pursuant to Section 5.3(b) within **** days after knowledge
of such infringement or misappropriation or, in the case of receipt of a notice letter sent by a
Third Party pursuant to the requirements of 21 U.S.C. § 355(b)(2)(A)(iv) or 355(j)(2)(A)(vii)(IV)
or under any analogous provisions, within **** before any statutory or regulatory deadline for
filing such suit, then OPKO will have the immediate right to initiate a suit or take other
appropriate action that it believes is reasonably required to prevent or abate actual or threatened
infringement or misappropriation of, or otherwise to protect or enforce the relevant OPKO Patent
Rights. TESARO and its Affiliates will join such suit if the relevant court would lack jurisdiction
if TESARO or such Affiliates were absent from such suit and TESARO and such Affiliates will execute
such legal papers and cooperate in the prosecution of such suit as may be reasonably requested by
OPKO; provided, that **** (including ****) incurred by **** and such Affiliates in connection with
such requested cooperation.
(d) Enforcement Against Other Infringement of OPKO Patent Rights. Except as
provided in Section 5.3(b), OPKO will have the sole right, but not the obligation, to initiate a
suit or take other appropriate action that it believes is reasonably required to prevent or abate
actual or threatened infringement or misappropriation of, or otherwise to protect or enforce, OPKO
Patent Rights during the Term.
(e) Right to Enforce Know-how. Responsibility for preventing or abating actual or
threatened infringement or misappropriation of, or otherwise protecting or enforcing OPKO Know-how
will be determined in the same manner as the right to enforce OPKO Patent Rights under paragraph
(b) and (c). The enforcing Party shall keep the other Party informed of the status of all
enforcement activities, and shall consider in good faith all comments of the other Party regarding
any aspect of such enforcement.
(f) Conduct of Certain Actions; Costs. The Party initiating suit under this
Section 5.3 will have the sole and exclusive right to select counsel for any suit initiated by it
pursuant to this Section. The initiating Party will assume and **** incurred in connection with any
litigation or proceedings initiated by it pursuant to this Section, including the **** selected by
it.
16
(g) Recoveries.
(i) If TESARO initiates suit as permitted in accordance with Section 5.3(b) or,
with respect to OPKO Know-how, in the same manner as set forth in Section 5.3(b), any
damages, settlements, accounts of profits, or other financial compensation actually paid to
TESARO by a Third Party based upon such suit, after deducting TESAROs actual out of pocket
expenses (including reasonable attorneys fees and expenses) incurred in pursuing such suit
(such net amount, the Recovery), will be treated as Net Sales, and will be subject
to the royalty payment obligations under Section 4.4 (provided that, for purposes of
calculating the applicable royalty rate, such Recovery will not be combined with any
calendar year Net Sales), with TESARO retaining the balance after such payment.
(ii) If OPKO initiates suit pursuant to Section 5.3(b) or with respect to OPKO
Know-how, in the same manner as set forth in Section 5.3(b), OPKO may retain any damages,
settlements, accounts of profits, or other financial compensation recovered from a Third
Party based upon such suit.
5.4. Patent Invalidity Claim. Each of the Parties will promptly notify the other
Party in the event of any legal or administrative action by any Third Party against an OPKO Patent
Right, or any certification filed pursuant to 21 U.S.C. § 355(b)(2)(A)(iv) or 355G)(2)(A)(vii)(IV)
or any notice under any analogous provisions, with respect to such Patent Rights, of which it
becomes aware, including any nullity, revocation, reexamination or compulsory license proceeding.
Responsibility for defending against any such action shall be determined in the same manner as
enforcement of the relevant Patent Rights pursuant to Section 5.3.
5.5. Patent Marking. TESARO agrees to comply with the patent marking statutes in
each country in which the Licensed Product is sold by TESARO or its Affiliates or Sublicensees.
ARTICLE VI
CONFIDENTIALITY
CONFIDENTIALITY
6.1.
Confidential Information. During the Term and for a period of **** after any
termination or expiration of this Agreement, each Party agrees to keep in confidence and not to
disclose to any Third Party, or use for any purpose, except pursuant to, and in order to carry out,
the terms and objectives of this Agreement (which, in the case of TESARO and its Affiliates and
Sublicensees, includes activities contemplated by the licenses granted in Sections 2.1) or as
otherwise specifically permitted under this Agreement, any Confidential Information of the other
Party. The terms of this Agreement will be considered Confidential Information of both Parties,
subject to permitted disclosures as set forth in this Article VI. The restrictions on the
disclosure and use of Confidential Information set forth in the first sentence of this Section 6.1
will not apply to any Confidential Information that:
(i) was known by the receiving Party prior to disclosure by the disclosing Party
hereunder (as evidenced by the receiving Partys written records or other competent
evidence);
(ii) is or becomes part of the public domain through no fault of the receiving
Party;
(iii) is disclosed to the receiving Party by a Third Party having a legal right to
make such disclosure without violating any confidentiality or non-use obligation that such
Third Party has to the disclosing Party and provided such Third Party is not disclosing such
information on behalf of the disclosing Party; or
17
(iv) is independently developed by personnel of the receiving Party who did not
have access to the Confidential Information (as evidenced by the receiving Partys written
records or other competent evidence).
In addition, if either Party is required to disclose Confidential Information of the other Party by
regulation, law or legal process, including by the rules or regulations of the United States
Securities and Exchange Commission or similar regulatory agency in a country other than the United
States or of any stock exchange or Nasdaq, such Party shall provide prior written notice and a copy
of such intended disclosure to such other Party if possible under the circumstances, will consider
in good faith the other Partys comments, will disclose only such Confidential Information of such
other Party as is required to be disclosed and will cooperate in the disclosing Partys efforts to
obtain a protective order or to limit the scope of the required disclosures. Notwithstanding
anything in this Agreement to the contrary, either Party may disclose to bona fide potential or
existing investors or lenders, potential acquirors/acquirees, and, in the case of TESARO, to
potential and existing sublicensees and collaborators, and to such Partys consultants and
advisors, the existence and terms of this Agreement to the extent necessary in connection with a
proposed equity or debt financing of such Party, or a proposed acquisition or business combination
or transaction, so long as such recipients are bound in writing to maintain the confidentiality of
such information.
6.2. Permitted Disclosures. Each Party agrees that it and its Affiliates will
provide or permit access to Confidential Information received from the other Party and such Partys
Affiliates and representatives only to the receiving Partys employees, consultants, advisors and
bona fide potential acquirors, and, in the case of TESARO as the receiving Party, to service
providers, investigators, Third Party contractors, potential and existing Sublicensees and
distributors, in each case who, in such Partys reasonable judgment, have a need to know such
Confidential Information to assist the receiving Party with the activities contemplated by this
Agreement (which, in the case of TESARO and its Affiliates and Sublicensees, includes activities
contemplated by the license granted in Sections 2.1) or in connection with a potential business
relationship or investment that would encompass Licensed Product, and who are subject to
obligations of confidentiality and non-use with respect to such Confidential Information similar to
the obligations of confidentiality and non-use of the receiving Party under Section 6.1. OPKO and
TESARO shall each remain responsible for any failure by its Affiliates, and its and its Affiliates
respective employees, consultants, advisors and permitted contractors, sublicensees and
distributors, to treat such Confidential Information as required under Section 6.1 (as if such
Affiliates, employees, consultants, advisors, contractors, sublicensees and distributors were
Parties directly bound to the requirements of Section 6.1). TESARO may also disclose Confidential
Information of OPKO to Regulatory Authorities and other governmental authorities, but solely in
connection with the activities contemplated by this Agreement.
6.3. Limitation on OPKO Disclosure of OPKO Know-how. During the Term of this
Agreement, OPKO will not disclose OPKO Know-how that is specific to Licensed Product or the
development, manufacture, commercialization or use of Licensed Product to any Third Party without
the express written consent of TESARO.
6.4. Publicity. Neither Party will issue a press release or public announcement
relating to the terms of this Agreement without the prior written approval of the other Party,
which approval shall not be unreasonably withheld or delayed, except that (i) either or both of the
Parties may issue a press release in the form attached as Exhibit E; (ii) a Party may issue
such press release or public announcement if the contents of such press release or public
announcement are consistent with a previously approved press release or have otherwise previously
been made public other than through a breach of this Agreement, and (ii) a Party may issue such a
press release or public announcement if required by applicable law, including by the rules or
regulations of the United States Securities and Exchange Commission (SEC) or similar regulatory
agency in a country other than the United States or of any stock exchange or Nasdaq; provided that
such Party complies with the notice and review provisions set forth in this Section. In no
18
event will OPKO make any public disclosure related to TESAROs activities under this Agreement
or related to the results generated by TESARO or any of its Affiliates or Sublicensees with respect
to Licensed Product without the prior written consent of TESARO except to the extent required by
applicable law. In the event OPKO is required by applicable law to publicly disclose any of the
results generated by TESARO or any of its Affiliates or Sublicensees or any information provided by
TESARO related to Licensed Product or either Party is required by applicable law to disclose the
terms of this Agreement, such Party will give the other Party at least two (2) business days prior
written notice, will provide to such other Party a copy of the required disclosure, will, if
requested by such other Party, to the extent permitted by applicable law, request confidential
treatment of any financial and other materials terms of this Agreement not previously disclosed
under this Section, and will consider in good faith any other comments of such other Party on such
public disclosure.
6.5. Publications. TESARO and its Affiliates and Sublicensees shall have the sole
right to publish the results of development, manufacture, commercialization and use of Licensed
Product during the Term.
6.6. Return of Confidential Information. Upon termination of this Agreement prior
to the end of the Term, the receiving Party shall, at the request of, and as directed by, the
disclosing Party, return or destroy Confidential Information of the disclosing Party in the
receiving Partys possession, and shall destroy any reports or notes in receiving Partys
possession t6 the extent containing the disclosing Partys Confidential Information, and any
electronic copies of any of the foregoing, provided that (i) the receiving Party may retain one
copy of Confidential Information of the disclosing Party for archival purposes, and (ii) neither
Party shall be required to return or destroy copies of the other Partys Confidential Information
stored on automatically created system back-up media.
ARTICLE VII
REPRESENTATIONS AND WARRANTIES; CERTAIN COVENANTS
REPRESENTATIONS AND WARRANTIES; CERTAIN COVENANTS
7.1. Mutual Representations. Each Party hereby represents and warrants to the other Party,
as of the Effective Date, as follows:
(a) It is duly organized and validly existing under the laws of its jurisdiction of
incorporation and has the corporate power and authority to execute and deliver this Agreement and
to perform its obligations hereunder.
(b) The execution, delivery and performance of this Agreement by such Party has been duly
and validly authorized and approved by proper corporate action on the part of such Party. Such
Party has taken all other action required by applicable law, its certificate of incorporation or
by-laws or any agreement to which it is a party or by which it or its assets are bound, to
authorize such execution, delivery and performance. Assuming due authorization, execution and
delivery on the part of the other Party, this Agreement constitutes a legal, valid and binding
obligation of such Party.
(c) The execution and delivery of this Agreement, and the performance as contemplated
hereunder, by such Party will not violate any applicable law.
(d) Neither the execution and delivery of this Agreement nor the performance hereof by
such Party requires such Party to obtain any permit, authorization or consent from any governmental
authority (except for any Regulatory Approvals, pricing or reimbursement approvals,
manufacturing-related approvals or similar approvals necessary for development, manufacture or
commercialization of Licensed Products), or from any other person, and such execution, delivery and
performance by such Party, including the granting of the licenses granted under this Agreement,
will not result in the breach of or give rise to any conflict, termination of, rescission,
renegotiation or acceleration under or trigger any
19
other rights under any agreement or contract to which such Party may be a party existing as of
the Effective Date.
(e) Neither Party nor any of its Affiliates has been debarred or is subject to debarment,
and OPKO has not used in any capacity in connection with the development or manufacture of Licensed
Product prior to the Effective Date, any person or entity who has been debarred pursuant to Section
306 of the United States Federal Food, Drug, and Cosmetic Act, or who is the subject of a
conviction described in such section.
7.2. OPKOs Representations and Warranties. OPKO hereby makes the following
representations and warranties to TESARO as of the Effective Date:
(a) OPKO has the right to grant to TESARO the rights and licenses described in this
Agreement.
(b) Exhibit C contains a complete and correct list of all existing OPKO Patent
Rights.
(c) To OPKOs knowledge, no Third Party is infringing any of the OPKO Patent Rights
identified on Exhibit C.
(d) To OPKOs knowledge, except as discussed with TESARO, the making, using or selling of
a Licensed Product will not infringe any Third Party Patent Rights.
(e) OPKO has not received any written notice of (i) any claim that any patent or trade
secret right owned or controlled by a Third Party would be infringed or misappropriated by the
manufacture, use, sale, offer for sale or importation of Licensed Products in the Field, or (ii)
any threatened claims or litigation seeking to invalidate or otherwise challenge the OPKO Patent
Rights or OPKOs rights therein.
(f) OPKOs rights to OPKO Patent Rights and OPKO Know-how are held free and clear of any
liens, security interests and similar encumbrances.
(g) None of the OPKO Patent Rights owned by OPKO are the subject of any pending
re-examination, opposition, interference or litigation proceedings.
(h) To OPKOs knowledge, there have been no inventorship or ownership challenges with
respect to any of the OPKO Compound Patent Rights.
(i) The OPKO Patent Rights that are pending patent applications as of the Effective Date
are being diligently prosecuted at the respective patent offices. To OPKOs knowledge, the OPKO
Patent Rights that are issued patents have been maintained properly and correctly and all
applicable fees have been paid on or before the due date for payment.
(j) There are no agreements pursuant to which a Third Party has licensed to OPKO any OPKO
Patent Rights or OPKO Know-how or pursuant to which OPKO or any of its Affiliates has otherwise
acquired any OPKO Patent Rights or OPKO Know-how from a Third Party other than the Asset Purchase
Agreement or other Third Party Agreements.
(k) A complete list of material agreements to which OPKO or any of its Affiliates is a
Party related to the development, manufacture, use or sale of Licensed Product or under which OPKO
may otherwise be required to make payments to Third Parties related to this Agreement is attached
as Exhibit F (the Third Party Agreements). OPKO will not amend, allow to terminate, or waive any
of its
20
rights or obligations under the Asset Purchase Agreement in a manner which would adversely
impact the rights licensed to TESARO under this Agreement, except as approved in writing in advance
by TESARO.
(l) To OPKOs knowledge, the research, development and manufacture of Licensed Product in
the Territory on or before the Effective Date has been conducted by OPKO and its Affiliates and its
subcontractors, in compliance (in all material respects) with all applicable laws.
(m) Neither OPKO nor its Affiliates has received written notice from any Regulatory
Authority threatening any proceedings with respect to the research, development or manufacture of
any Licensed Product in the Field in the Territory.
(n) To OPKOs knowledge, OPKO has not intentionally withheld any material information
relating to the subject matter of this Agreement.
7.3. No Warranty. EXCEPT AS OTHERWISE EXPRESSLY SET FORTH IN THIS AGREEMENT,
NEITHER PARTY HERETO MAKES ANY REPRESENTATIONS AND NEITHER PARTY EXTENDS ANY WARRANTIES OF ANY
KIND, EITHER EXPRESS, IMPLIED, STATUTORY OR OTHERWISE, WITH RESPECT TO THE SUBJECT MATTER OF THIS
AGREEMENT (INCLUDING ANY LICENSED PRODUCT), INCLUDING ANY WARRANTY OF MERCHANTABILITY,
NONINFRINGEMENT, OR FITNESS FOR A PARTICULAR PURPOSE. EXCEPT AS EXPRESSLY PROVIDED IN THIS
AGREEMENT, OPKO MAKES NO WARRANTY OR REPRESENTATION AS TO THE VALIDITY OR SCOPE OF THE OPKO PATENT
RIGHTS OR OPKO KNOW HOW, OR THAT ANY LICENSED PRODUCT WILL BE FREE FROM AN INFRINGEMENT ON PATENTS
OR OTHER INTELLECTUAL PROPERTY RIGHTS OF THIRD PARTIES, OR THAT NO THIRD PARTIES ARE IN ANY WAY
INFRINGING OR NOT INFRINGING THE OPKO PATENT RIGHTS OR OPKO KNOW HOW COVERED BY THIS AGREEMENT.
TESARO DISCLAIMS ANY REPRESENTATION OR WARRANTY THAT THE DEVELOPMENT, MANUFACTURE AND
COMMERCIALIZATION OF LICENSED PRODUCT PURSUANT TO THIS AGREEMENT WILL BE SUCCESSFUL OR THAT, IF
COMMERCIALIZED, ANY PARTICULAR SALES LEVEL WILL BE ACHIEVED.
ARTICLE VIII
INDEMNIFICATION
INDEMNIFICATION
8.1. Indemnification by TESARO. TESARO will indemnify, hold harmless, and defend
OPKO, its Affiliates, and their respective directors, officers, employees and agents (the OPKO
Indemnitees) from and against any and all damages, liabilities, costs, expenses and amounts paid
in settlement (collectively, Losses) incurred in connection with any Third Party claim arising
out of or resulting from, directly or indirectly; (i) any breach of, or inaccuracy in, any
representation or warranty made by TESARO in this Agreement, or any breach or violation of any term
of this Agreement by TESARO; (ii) the negligence or willful misconduct of TESARO, its Affiliates
and their respective Sublicensees, and their respective directors, officers, employees and agents;
and (iii) the research, development, manufacture, commercialization, or use of Licensed Product by
TESARO and its Affiliates and Sublicensees in the Territory in the Field under this Agreement.
Notwithstanding the foregoing or anything in this Agreement to the contrary, TESARO will have no
obligation to indemnify the OPKO Indemnitees to the extent that the Losses arise out of or result
from, directly or indirectly, any breach of, or inaccuracy in, any representation or warranty made
by OPKO in this Agreement; any breach or violation of any term of this Agreement by OPKO; the
negligence or willful misconduct of any of the OPKO Indemnitees or any other Losses as to which
OPKO is obligated to indemnify TESARO under Section 8.2.
8.2. Indemnification by OPKO. OPKO will indemnify, hold harmless, and defend
TESARO, its Affiliates and their respective directors, officers, employees and agents (the TESARO
Indemnitees)
21
from and against any and all Losses incurred in connection with any Third Party Claim
arising out of or resulting from, directly or indirectly, (i) any breach of, or inaccuracy in, any
representation or warranty made by OPKO in this Agreement, or any breach or violation of any term
of this Agreement by OPKO;
(ii) the negligence or willful misconduct of any OPKO Indemnitee; (iii) the research,
development, manufacture or use of Licensed Product by or on behalf of OPKO or any of its
Affiliates prior to commencement of the Term; or (iv) the research, development, manufacture,
commercialization, or use of Licensed Product by OPKO or any of its Affiliates or licensees (other
than TESARO) or any other activities of OPKO and its Affiliates and licensees (other than TESARO)
outside the Field. Notwithstanding the foregoing, or anything in this Agreement to the contrary,
OPKO will have no obligation to indemnify the TESARO Indemnitees for any Losses as to which TESARO
is obligated to indemnify OPKO under Section 8.1.
8.3. Indemnification Procedure. In the event of any such claim against any TESARO
Indemnitee or OPKO Indemnitee (individually, an Indemnitee), the indemnified Party shall promptly
notify the other Party in writing of the claim and the indemnifying Party shall manage and control,
at its sole expense, the defense of the claim and its settlement. The indemnified Party will
cooperate with the indemnifying Party and may, at the indemnifying Partys option and expense, be
represented in any such action or proceeding. The indemnifying Party will not be liable for any
settlements, litigation costs or expenses incurred by any Indemnitee without the indemnifying
Partys prior written authorization. Notwithstanding the foregoing, if the indemnifying Party
believes that any of the exceptions to its obligation of indemnification of the Indemnitees set
forth in this Article 8 may apply, the indemnifying Party will promptly notify the Indemnitees, who
shall then have the right to be represented in any such action or proceeding by separate counsel at
their expense; provided that the indemnifying Party will be responsible for payment of such
expenses if the Indemnitees are ultimately determined to be entitled to indemnification from the
indemnifying Party.
8.4. Limitation of Liability. NEITHER PARTY HERETO WILL BE LIABLE FOR SPECIAL,
INCIDENTAL, CONSEQUENTIAL OR PUNITIVE DAMAGES ARISING OUT OF THIS AGREEMENT OR THE EXERCISE OF ITS
RIGHTS HEREUNDER, INCLUDING LOST PROFITS ARISING FROM OR RELATING TO ANY BREACH OF THIS AGREEMENT,
REGARDLESS OF ANY NOTICE OF SUCH DAMAGES, EXCEPT AS A RESULT OF A PARTYS WILLFUL MISCONDUCT.
NOTHING IN THIS SECTION 8.4 IS INTENDED TO LIMIT OR RESTRICT THE INDEMNIFICATION RIGHTS OR
OBLIGATIONS OF EITHER PARTY.
8.5. Insurance. During the Term and for a period of at least **** years after the
last commercial sale of a Licensed Product in the Field under this Agreement, TESARO will maintain
insurance, with a reputable, solvent insurer in an amount appropriate for its business and products
of the type that are the subject of this Agreement, and for its obligations under this Agreement,
including, commencing immediately prior to the first human clinical trial, product and clinical
trial liability insurance of at least **** per occurrence and **** in the aggregate on a
worldwide basis.
ARTICLE IX
TERM AND TERMINATION
TERM AND TERMINATION
9.1. Term. This Agreement will become effective as of the Effective Date, and will
continue in full force and effect until the last to expire Royalty Term and Japan Income Sharing
Term, unless earlier terminated in accordance with this Article IX (Term). Upon
expiration of the Term under the preceding sentence (but not earlier termination of this Agreement)
the licenses granted to TESARO under Section 2.1 will convert to perpetual, fully paid-up,
non-royalty-bearing licenses with the same scope as set forth in such Section.
9.2. Termination for Convenience. TESARO will have the right to terminate this
Agreement at any time and for any reason upon at least three (3) months prior written notice to
OPKO.
22
9.3. Termination for Cause. This Agreement may be terminated at any time during
the Term upon written notice by either Party if the other Party is in material breach of its
obligations hereunder, and has not cured such material breach within sixty (60) days after written notice describing the
nature of such material breach is provided to the breaching Party.
9.4. OPKO Termination. To the extent permitted by applicable law, OPKO may
terminate this Agreement by giving written notice of termination to TESARO within thirty (30) days
of the filing of bankruptcy or bankruptcy of TESARO or the making by TESARO of any assignment for
the benefit of creditors. Termination shall be effective upon the date specified in such notice.
9.5. Effect of Termination.
(a) Pre-Termination Obligations; Transfer of Information and Filings. Upon the
termination of this Agreement for any reason, nothing herein shall be construed to release either
party from any obligation that matured prior to the effective date of such termination. TESARO
shall remain obligated to provide an accounting for and to pay Royalties earned. In the event of
termination, (i) the licenses granted hereunder shall terminate; (ii) TESARO shall have no further
right under OPKO Patent Rights or OPKO Know-how to develop, manufacture or market the Licensed
Product or any product containing Licensed Product for use in the Field, or otherwise to use the
OPKO Patent Rights or OPKO Know How; (iii) all rights granted hereunder shall revert to OPKO for
the benefit of OPKO; and (iv) TESARO shall, as promptly as practicable, transfer to OPKO or OPKOs
designee: (a) possession and ownership of all governmental or regulatory correspondence,
conversation logs, filings and approvals (including all Marketing Approvals and pricing and
reimbursement approvals) relating to the development, manufacture or commercialization of the
Licensed Product in the Field and all product trademarks then being used in connection with
Licensed Product, other than TESAROs corporate trademarks; and (b) all safety data and other
adverse event data in TESAROs possession or Control. In addition, OPKO shall have the right to
purchase all API and Licensed Product in TESAROS possession or control at **** or Licensed Product
(other than **** pursuant to this Agreement, which will be ****). Notwithstanding the foregoing,
TESARO shall be entitled to sell any completed inventory of Licensed Product which remain on hand
as of the date of the termination, and to sell new inventory to the extent necessary to satisfy its
contractual and legal obligations, so long as TESARO pays to OPKO the royalties applicable to said
subsequent sales in accordance with the terms and conditions as set forth in this Agreement;
provided that no sales shall be permitted after the expiration of six (6) months after the date of
termination. TESARO will execute all documents and take all such further actions, as may be
reasonably requested by OPKO in order to give effect to the preceding sentences as soon as
practicable.
(b) License Grant. In the event of termination of this Agreement by OPKO under
Section 9.3 or 9.4 or termination by TESARO under Section 9.2, TESARO will be deemed to have
granted to OPKO a royalty-bearing (but solely to the extent set forth below), worldwide, exclusive,
sublicensable, license under any TESARO Improvement Patent Rights and TESARO Improvement to the
extent necessary or reasonably useful to manufacture, market, sell or use Licensed Product in the
Field in the Territory and solely for such purpose. Except in the event of termination by OPKO
under Section 9.3 or 9.4, OPKO will pay to TESARO a royalty on the sale of any Licensed Product in
the Field that incorporates a TESARO Improvement and is Covered by a Valid Claim of a TESARO
Improvement Patent Right, as follows:
| Development Stage as of Date of Termination | Royalty Rate | |
After the first NDA filing of a Licensed Product in the US or EU but prior to first commercial sale of
a Licensed Product in the US or EU
|
**** | |
After first commercial sale of a Licensed Product in the US or EU
|
**** |
23
In addition, in the event TESARO or any of its Affiliates or Sublicensees is required to make
payments to any Third Party by reason of the licenses granted to OPKO under this paragraph (b) and
based on the development, manufacture or sale of Licensed Product by or on behalf of OPKO or any of
its Affiliates or sublicensees, OPKO will pay such amounts due by TESARO or any of its Affiliates
or Sublicensees to such Third Party by reimbursing TESARO or paying such amounts directly to such
Third Party, as directed by TESARO, in each case based on supporting documentation provided by
TESARO. OPKO may elect not to accept the grant of the license to TESARO Improvement Patent Rights
upon thirty (30) days written notice to TESARO from the date of termination.
9.6. Survival. Any expiration or termination of this Agreement will be without
prejudice to the rights of either Party against the other accrued or accruing under this Agreement
prior to expiration or termination, including payment obligations arising prior to such expiration
or termination. The provisions of Articles VI, VIII, IX, X and XI will survive any expiration or
termination of this Agreement and all other provisions contained in this Agreement that by their
explicit terms survive expiration or termination of this Agreement, will survive. Except as set
forth in this Article IX, upon termination or expiration of this Agreement all other rights and
obligations of the Parties under this Agreement terminate.
ARTICLE X
DISPUTE RESOLUTION
DISPUTE RESOLUTION
10.1. Continuance of Rights and Obligations During Pendency of Dispute Resolution.
If there are any disputes in connection with this Agreement, including disputes related to
termination of this Agreement under Article IX, all rights and obligations of the Parties shall
continue until such time as any dispute has be resolved in accordance with the provisions of this
Article X.
10.2. Referral of Unresolved Matters to Senior Executives. In the event that the Parties
are unable to resolve a dispute within fifteen (15) days from the date such dispute is first
brought to the other Partys attention, the matter shall be referred to a senior executive of each
Party to be resolved by negotiation in good faith as soon as is practicable but in no event later
than thirty (30) days after referral.
10.3. Arbitration. Any dispute, controversy or claim arising out of or relating to
this Agreement which the Parties have not resolved under Section 10.2, will be decided by
arbitration in accordance with the Rules of the American Arbitration Association for Commercial
Arbitration in effect at the time the dispute arises, unless the Parties hereto mutually agree
otherwise. To the extent such rules are inconsistent with this provision, this provision will
control. The following rules will apply to any such arbitration:
(a) Any demand for arbitration must be made in writing to the other Party.
(b) There will be three arbitrators, one of whom shall be appointed by each party and a
third of whom shall be the chairman of the panel and be appointed by mutual agreement of the two
arbitrators appointed by the Parties. If the two arbitrators cannot agree on the appointment of the
third arbitrator within thirty (30) days, then the AAA shall select the arbitrator. Any arbitration
involving patent rights, other intellectual property rights or intellectual property will be heard
by arbitrators who are expert in such areas.
(c) The arbitration will be held in the State of Delaware, or such other place as the
Parties agree. The arbitrators will apply the substantive law of the State of Delaware in
accordance with Section 11.1, without regard to conflicts of laws and except that the
interpretation and enforcement of this arbitration provision will be governed by the Federal
Arbitration Act, 9 U.S.C. Section 1 et. seq.
(d) Neither Party will have the right independently to seek recourse from a court of law
or other authorities in lieu of arbitration, but each Party has the right before or during the
arbitration to seek and obtain from the appropriate court provisional remedies to avoid irreparable
harm, maintain the
24
status quo or preserve the subject matter of the arbitration. There shall be a stenographic
record of the proceedings. The decision of the arbitrators will be final and binding upon both
Parties. The arbitrators will render a written opinion setting forth findings of fact and
conclusions of law.
(e) The expenses of the arbitration will be borne by the Parties in proportion as to which
each Party prevails or is defeated in arbitration. Each Party will bear the expenses of its counsel
and other experts.
10.4. Equitable Relief. Notwithstanding anything to the contrary, each of the
Parties hereby acknowledges that a breach of their respective obligations under this Agreement may
cause irreparable harm and that the remedy or remedies at law for any such breach may be
inadequate. Each of the Parties hereby agrees that, in the event of any such breach, in addition to
all other available remedies hereunder, the non-breaching Party shall have the right, through the
arbitration process described in Section 10.3, to seek equitable relief to enforce the provisions
of this Agreement.
ARTICLE XI
MISCELLANEOUS
MISCELLANEOUS
11.1. Governing Law and Jurisdiction. The validity, construction and performance
of this Agreement will be governed by and construed in accordance with the substantive laws of the
State of Delaware excluding any conflicts or choice of law rule or principle that might otherwise
refer construction or interpretation of this Agreement to the substantive law of another
jurisdiction.
11.2. Force Majeure. Neither Party will be held liable or responsible to the other Party
nor be deemed to have defaulted under or breached this Agreement for failure or delay in fulfilling
or performing any term, other than an obligation to make payments hereunder, when such failure or
delay is caused by or results from fire, floods, embargoes, government regulations, prohibitions or
interventions, war, acts of war (whether war be declared or not), insurrections, riots, civil
commotions, acts of God or any other cause beyond the reasonable control of the affected Party to
anticipate, prevent, avoid or mitigate (a Force Majeure Event); provided that (i) the
affected Party provides prompt written notice to the other Party of such failure or delay, (ii) the
affected Party uses Commercially Reasonable Efforts to mitigate the effects of the Force Majeure
Event, and (iii) the affected Party immediately resumes performance upon cessation of the Force
Majeure Event. Notwithstanding the foregoing, any failure or delay in fulfilling a term shall not
be considered a result of a Force Majeure Event if it arises from a failure of TESARO or OPKO to
comply with applicable laws.
11.3. Further Assurances. Each Party hereto agrees to perform such acts, execute
such further instruments, documents or certificates, and provide such cooperation in proceedings
and actions as may be reasonably requested by the other Party in order to carry out the intent and
purpose of this Agreement.
11.4. Notices. Any notice required or permitted to be given under this Agreement
will be in writing and will be deemed to have been properly given if delivered in person by a
internationally recognized overnight courier, or by fax (and promptly confirmed by overnight
courier), to the addresses given below or such other addresses as may be designated in writing by
the Parties from time to time during the Term.
In the case of TESARO:
TESARO, Inc.
309 Waverley Oaks Rd., Suite 101
Waltham, MA 02452
Attention: Chief Financial Officer
Fax No.: 339-469-8966
309 Waverley Oaks Rd., Suite 101
Waltham, MA 02452
Attention: Chief Financial Officer
Fax No.: 339-469-8966
25
With a copy to:
Anne Marie Cook
Choate, Hall & Stewart LLP
Two International Place
Boston, MA 02110
Fax No.: 617-248-4000
Choate, Hall & Stewart LLP
Two International Place
Boston, MA 02110
Fax No.: 617-248-4000
In the case of OPKO:
OPKO Health Inc.
4400 Biscayne Blvd.
Miami, FL 33137
Attention: Executive Vice President
Fax No.: 305-575-6444
4400 Biscayne Blvd.
Miami, FL 33137
Attention: Executive Vice President
Fax No.: 305-575-6444
With a copy to: Deputy General Counsel
11.5. Assignment. This Agreement may not be assigned or otherwise transferred by
either Party, without the written consent of the other Party such consent not to be unreasonably
withheld, conditioned or delayed; provided, however, that either Party may, without such consent,
assign this Agreement, in whole or in part, (i) to any of its Affiliates, and (ii) to a Third Party
successor or purchaser of all or substantially all of its business or assets to which this
Agreement relates, whether in a merger, sale of stock, sale of assets or other similar transaction,
provided that, (i) the Third Party successor or purchaser provides written notice to the other
Party that such Third Party agrees to be bound by the terms of this Agreement, and (ii) OPKO will
not assign this Agreement unless the assignee is also assigned ownership owns or Controls of the
OPKO Patent Rights and OPKO Know-how. Any purported assignment in violation of this Section 11.5
will be void. Any permitted assignee shall assume all obligations of its assignor under this
Agreement.
11.6. Affiliate Performance. Any obligation of TESARO under or pursuant to this
Agreement may be satisfied, met or fulfilled, in whole or in part, at TESAROs sole and exclusive
option, either by TESARO directly or by any Affiliate or Sublicensee of TESARO that TESARO causes
to satisfy, meet or fulfill such obligation, in whole or in part.
11.7. Amendment. The Parties hereto may amend, modify or alter any of the
provisions of this Agreement, but only by a written instrument duly executed by both Parties
hereto.
11.8. Entire Agreement. This Agreement, along with all schedules and exhibits
attached hereto, contains the entire understanding of the Parties with respect to the subject
matter hereof and supersedes all prior agreements, whether written or oral. Each Party confirms
that it is not relying on any representations, warranties or covenants of the other Party except as
specifically set out in this Agreement.
11.9. No Benefit to Third Parties. The provisions of this Agreement are for the
sole benefit of the Parties and their successors and permitted assigns, and they shall not be
construed as conferring any rights in any other Persons.
11.10. Waiver. The failure of a Party to enforce at any time for any period any of
the provisions of this Agreement will not be construed as a waiver of such provisions or of the
rights of such Party thereafter to enforce each such provision.
11.11. No Implied Licenses. Except as expressly and specifically provided under
this Agreement, the Parties agree that neither Party is granted any implied rights to or under any
of the other
26
Partys current or future patents, trade secrets, copyrights, moral rights, trade or service
marks, trade dress, or any other intellectual property rights.
11.12. Relationship of the Parties. The Parties agree that their relationship
established by this Agreement is that of independent contractors. Furthermore, the Parties agree
that this Agreement does not, is not intended to, and shall not be construed to, establish a
partnership or joint venture, and nor shall this Agreement create or establish an employment,
agency or any other relationship. Except as may be specifically provided in this Agreement, neither
Party shall have any right, power or authority, nor shall they represent themselves as having any
authority to assume, create or incur any expense, liability or obligation, express or implied, on
behalf of the other Party, or otherwise act as an agent for the other Party for any purpose.
11.13. Severability. If any provision of this Agreement is held unenforceable by a
court or tribunal of competent jurisdiction in a final unappealable order because it is invalid or
conflicts with any law of any relevant jurisdiction, then such provision will be inoperative in
such jurisdiction and the remainder of this Agreement shall remain binding upon the Parties hereto.
11.14. Interpretation.
(a) General. Unless the context of this Agreement otherwise requires, (a) words of
one gender include the other gender; and (b) words using the singular or plural number also include
the plural or singular number, respectively. Whenever this Agreement refers to a number of days,
unless otherwise specified, such number shall refer to calendar days.
(b) Other Definitional and Agreement References. References to any agreement,
contract, statute, act, or regulation are to that agreement, contract, statute, act, or regulation
as amended, modified or supplemented from time to time in accordance with the terms hereof and
thereof.
(c) Capitalization. Any capitalized terms used in any Exhibit or Schedule but not
otherwise defined therein, shall have the meaning as defined in this Agreement.
(d) Date References. References from or through any date mean, unless otherwise
specified, from and including or through and including, respectively.
(e) Schedules and Exhibits. All Schedules and Exhibits annexed hereto or referred
to herein are hereby incorporated in and made a part of this Agreement as if set forth in full
herein.
(f) Person References. References to any Person include the successors and
permitted assigns of that Person.
(g) References to Parts of this Agreement. References to Articles, Sections,
Schedules, and Exhibits are to Articles, Sections, Schedules, and Exhibits of this Agreement unless
otherwise specified.
(h) Other Definitional and Interpretative Provisions. The words hereof, herein
and hereunder and words of like import used in this Agreement shall refer to this Agreement as a
whole and not to any particular provision of this Agreement. Whenever the words include,
includes or including are used in this Agreement, they shall be deemed to be followed by the
words without limitation, whether or not they are in fact followed by those words or words of
like import. The word or is used in the inclusive sense (and/or). Writing, written and
comparable terms refer to printing, typing and other means of reproducing words (including
electronic media) in a visible form.
(i) Headings. The Article and Section headings contained in this Agreement are for
reference purposes only and shall not affect in any way the meaning or interpretation of this
Agreement.
27
(j) Expenses. Except as otherwise expressly provided in this Agreement, each Party
shall pay the fees and expenses of its respective lawyers and other experts and all other expenses
and costs incurred by such Party incidental to the negotiation, preparation, execution and delivery
of this Agreement.
11.15. Counterparts. This Agreement may be executed in any number of counterparts
(including by facsimile), each of which shall be deemed an original, but all of which together
shall constitute one and the same document.
[Signature Page Follows]
28
IN WITNESS WHEREOF, TESARO and OPKO have caused this Agreement to be duly executed by their
authorized representatives under seal, in duplicate on the Effective Date.
| TESARO, Inc. |
||||
| By: | ||||
| Name: | ||||
| Title: | ||||
| OPKO Health, Inc. |
||||
| By: | ||||
| Name: | ||||
| Title: | ||||
29
Exhibit A
Description of SCH 619734 (Rolapitant)
Chemical Structure of Rolapitant:
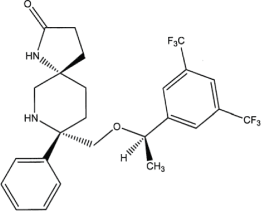
Exhibit B
Description of SCH 900978
Chemical Structure of SCH 900978:
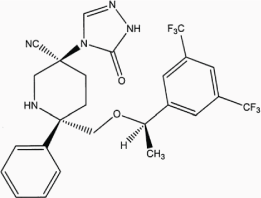
Exhibit C
OPKO Patent Rights
OPKO Patent Rights
[Attached]
| Monday, November 22, 2010 | Page 1 of 28 | |||
| Patent Status by Division | ||||
| Division: OPKO OPKO |
| Docket | Status | Application Number | Filing Date | ||||||||||||||||||||||||
| Country | SubCase | Case Type | Division Reference | Patent Number | Issue Date | Next Action(s) | Due Date(s) | ||||||||||||||||||||
**** |
|||||||||||||||||||||||||||
Monday, November 22, 2010
|
Report Selection | Page 2 of 28 Record Count: 187 |
| Sort Order: by Division | Print Remarks?: No | ||||
| Print Inventors?: No | |||||
| Print Abstract: No | |||||
| Actions Due: All |
| Filing Date: | From: | To: | ||||
Docket Number:
|
Status Code: All | |||||
Division: OPKO
|
Case Type(s): | Status(es): | ||||
Agent: |
||||||
Attorney: |
||||||
Assignee: |
||||||
Country: |
||||||
Area: |
||||||
Inventor: |
||||||
Exhibit D
Technology Transfer Plan
Technology Transfer Plan
[Attached]
TECHNOLOGY TRANSFER PLAN
This Technology Transfer Plan is an exhibit to the Exclusive License Agreement entered into between
TESARO, Inc. (TESARO) and OPKO Health, Inc. (OPKO) (the Agreement), and is incorporated by
reference into the Agreement. Capitalized terms used in this Technology Transfer Plan will have the
meaning set forth in the Agreement.
Part A -General
1. Technology Transfer Services. OPKO will transfer to TESARO (or TESAROs designees) all
OPKO Know How and related technical information, and provide such support, as is reasonably
necessary to enable TESARO to assume responsibility for the research, formulation, development,
testing and manufacture of Licensed Product, and, during the period commencing on the Effective
Date and continuing until the later of the completion of all Technical Transfer Services (as
defined below) or **** from the Effective Date (such period being hereafter referred to as the
Transfer Period), will provide reasonable ongoing assistance to TESARO in connection with such
transfer and use of the OPKO Know-how. In connection with the foregoing, OPKO will perform the
activities set forth in Parts Band C of this Technology Transfer Plan (the Technology Transfer
Services). In addition, during the Transfer Period, OPKO will make its personnel reasonably
available to TESARO to respond to questions related to the OPKO Know-how in connection with any of
the activities described in this Technology Transfer Plan, and will provide such ongoing support
and assistance as TESARO may reasonably request in the transition of development and manufacturing
responsibility for Licensed Products to TESARO. TESARO acknowledges that OPKO and OPKO personnel
were not involved in the discovery, manufacture, formulation, sourcing, research or development of
the Licensed Product or any API and have only gained information relating to the Licensed Product
in connection with the Asset Purchase Agreement and its research and development efforts undertaken
since the consummation of the Asset Purchase Agreement in November 2009, much of which has been
undertaken through the assistance of Third Party consultants. OPKO intended to use Third Parties
for, and therefore had not engaged in, the development or formulation of dosage forms or the
manufacture of drug product or API in support of the clinical development program or
commercialization of Licensed Product. Accordingly, OPKOs efforts, support and assistance and
TESAROs expectations under this Technology Transfer Plan must be considered in light of OPKO s
limited level of expertise, knowledge and familiarity with Licensed Product. Additionally, in
making the decision to enter into the Exclusive License Agreement, TESARO has conducted its own
independent investigation, review and analysis of the Licensed Product, OPKO Patent Rights and OPKO
Know-how, and has had complete access to all of OPKOs files, information, materials and data, and
records relating to the Licensed Product. In connection with the foregoing, at the request of
TESARO, OPKO will seek the assistance of Merck & Co., Inc. to the extent such support continues to
be available under Section 2.5 of the Asset Purchase Agreement, and any other Third Party support,
as specified in paragraph 2 of this Part A. To the extent information, data or materials referred
to in this Technology Transfer Plan are not in the possession of OPKO, or cannot be obtained from
Merck under the Asset Purchase Agreement pursuant to OPKOs rights thereunder, OPKO will have no
obligations hereunder or in the Agreement to provide or transfer such requested information, data
or materials. Notwithstanding anything herein to the contrary, this Technology Transfer Plan will
not be considered a limitation on, or a narrowing of, the obligations of either Party under the
Agreement.
2. Third Party Support. If any materials or information to be provided under this
Technology Transfer Plan or otherwise under the Agreement are in the possession or control of Merck
& Co., Inc. or any other Third Party who provided services to OPKO, OPKO will use Commercially
Reasonable Efforts to obtain such materials and information from Merck & Co., Inc. or such other
Third Party, as the case may be. In the case of materials and information in the possession or
control of Merck & Co., Inc., Commercially Reasonable Efforts under the preceding sentence will
include an obligation on the part of
1
OPKO to enforce its rights under the Asset Purchase Agreement. With respect to any provision under
this Technology Transfer Plan requiring OPKO to provide support or information from, or access to,
personnel, OPKO will, at the request of TESARO, arrange for, and facilitate, direct communication
between TESARO and any Third Party who was responsible for generating or implementing the
applicable OPKO Know-how. In particular, and especially with respect to the development,
implementation, transfer, provision or explanation of production manufacturing or formulation
processes for API or drug substance (for which OPKO has no direct knowledge), OPKO will, within ten
(10) days of the Effective Date, send written notice to Merck & Co., Inc. under which OPKO shall
specify TESARO as its designee under Section 2.5 of the Asset Purchase Agreement and authorizing
Merck & Co., Inc. to provide information, support and assistance to TESARO to the same extent as
available to OPKO under Section 2.5 of the Asset Purchase Agreement.
3. Technical Transfer Team. Commencing as of the Effective Date, the Parties will form a
technical transfer team (the Technical Transfer Team) comprised of the functions and individuals
identified below to coordinate and oversee the Technology Transfer Services.
| OPKO Representatives | ||||
| Functional Area Represented | Role | Initial Designee | ||
Team Leader
|
Act as primary interface with respect to OPKOs technology transfer activities | **** | ||
Regulatory
|
Implement transfer of the INDs, IMPDs and correspondence with health authorities | **** | ||
Clinical Research
|
Address questions related to completed clinical studies and those under planning or in start-up; oversee transfer of all clinical data (including but not limited to efficacy, safety, PK, ECG and pharmacovigilence), study documentation, safety reports, advisory meeting minutes, and inventoried biospecimens | **** | ||
Chemistry, Manufacturing,
and Controls
|
Oversee transfer of all pharmaceutical development data, technical and manufacturing documentation and inventoried non-GMP and GMP materials (Role is inclusive of API, starting materials, raw materials, retains, and stability programs in OPKOs possession or control) | **** |
2
| OPKO Representatives | ||||
| Functional Area Represented | Role | Initial Designee | ||
Preclinical Research
|
Address questions related to completed nonclinical studies and those under planning or in start-up; oversee transfer of all nonclinical data, study documentation and inventoried specimens (Role is inclusive of all toxicology, pharmacology, and pharmacokinetic and other preclinical activities) | **** | ||
Analytical Methods
|
Oversee transfer of all clinical, nonclinical and pharmaceutical analytical method development reports, final SOPs and associated reference standards | **** | ||
Quality
|
Oversee transfer of all quality audit and inspection reports, quality release documentation and other all associated quality memorandums in support of completed and planned development activities | **** | ||
General
|
Oversee transfer of all agreements, if any, to be assigned; transfer of any general program information and commercial information; and transfer of project team meeting minutes | **** | ||
Commercial
|
Oversee transfer of all market survey data and reports | **** | ||
IT (electronic files)
|
Information transfer | **** | ||
Patents
|
Oversee transfer of OPKO Patent Rights | **** |
3
| TESSARO Representatives | ||||
| Functional Area Represented | Role | Initial Designee | ||
Team Leader
|
Act as primary interface with respect to TESARO activities under Technology Transfer Plan | **** | ||
Regulatory
|
Receipt of IND and other regulatory docs | **** | ||
Clinical Research
|
Oversee receipt of technology related to clinical development | **** | ||
Chemistry, Manufacturing
and Controls
|
Oversee receipt and implementation of technology related to TESAROs CMC efforts and activities | **** | ||
Preclinical Research
|
Oversee receipt of technology transfer related to preclinical research activities | **** | ||
Analytical Methods
|
Act as primary interface with respect to transfer of analytical methods | **** | ||
Quality
|
Act as primary interface with respect to quality matters | **** | ||
General
|
Oversee transfer of all agreements, if any, to be assigned; transfer of any general program information and commercial information; and transfer of project team meeting minutes | **** | ||
Commercial
|
Act as primary interface | **** | ||
IT
|
Oversee information transfer | **** | ||
Patents
|
Oversee transfer of OPKO Patent Rights | **** |
Either Party may replace its representatives on the Technical Transfer Team, provided that the OPKO
representatives on the Technical Transfer Team will have comparative level of expertise, knowledge
and familiarity with Licensed Product to the listed representative.
The responsibilities of the Technical Transfer Team will include, but not be limited to the
following:
| a. | Establish a complete and reasonably detailed accounting of all materials, samples, documents, data, contracts, CD/DVDs and other electronic files that constitute the technical information embodying the OPKO Know-how, and assist in the complete and accurate transfer of all items to TESARO and/or any of TESAROS designees. Provide reasonable explanation to TESARO and/or any of TESAROS designees how items are related, filed, and what supportive software programs are required to enable any of the electronic files and data sets. |
| b. | Facilitate the reasonable assistance of OPKOs then current employees and reasonable access to its other internal resources and to Third Parties who generated or possess or control OPKO Know-how, to provide TESARO and/or any of TESAROs designees with a reasonable level of technical assistance and consultation in connection with the transfer of the OPKO Know- |
4
| how to TESARO and/or any of TESAROS designees, including the provision and explanation, upon request, to TESARO and/or any of its designees of all relevant technology, materials, reports, data, documents and materials describing or embodying the OPKO Know How. |
| c. | Facilitate the provision and explanation to TESARO and/or any of TESAROs designees, of all production outlines, materials sourcing, specifications, and testing, standard testing requirements (release, in process, characterization and stability), standard operating procedures (e.g. analytical testing, equipment cleaning), technology, documents (e.g. Certificates of Analysis, Specifications, technical reports, development reports and memorandums, Material Safety Data Sheets, qualification and validation reports, master manufacturing batch records, executed batch records), data, notebooks or other information that constitutes the OPKO Know-how for manufacture of starting materials, API and Licensed Product and intermediates of any of the foregoing. |
| d. | Facilitate the development and implementation of a technology transfer protocol for the transfer of the manufacturing process (including in-process methods) and formulation process for API, final drug substance and final drug product for the Licensed Products to TESARO and/or its designees. |
| e. | Implement transfer of clinical drug assay methodologies and know-how for the Licensed Products, including parent and metabolites, inventoried samples and completed or partial analyses (e.g. toxicokinetics, pharmarcokinetics) to TESARO and/or its designees. |
| f. | Implement transfer of all regulatory filings and sponsor of the INDs as promptly as practicable following the Effective Date. |
| g. | Establish a plan for and implement transfer of all electronic data and confirmation of data integrity and completeness and accuracy following transfer. |
| h. | Introduce TESARO and/or any of TESAROS designees, at TESAROs request, to consultants, contractors or other vendors currently engaged or involved in future planning activities related to the Licensed Products. |
| i. | Implement transfer to TESAROs designee all patent files related to OPKO Patent Rights in accordance with Section 5.1 of the License Agreement as necessary to allow TESARO to assume prosecution and maintenance of such OPKO Patent Rights without any loss of rights in the transition. |
4. Costs. **** associated with technology transfer activities to be provided under this
Technology Transfer Plan, including, but not limited to the ****. To the extent any Technology
Transfer Services to be provided under this Section require external resources, including
consultation with Third Party consultants, **** of such external resources, provided that such
activities and costs are expressly set forth in this Technology Transfer Plan or are otherwise
approved in writing in advance by ****.
Part B -Activities.
The activities to be performed by OPKO under this Technology Transfer Plan, by technical area, are
as follows:
5
| Function | Service to be Provided by OPKO | Comments | ||||
Technical Operations
|
OPKO shall provide the reasonable assistance of OPKOs then current employees and reasonable access to its other internal resources to provide TESARO (and/or TESAROs designees)with a reasonable level of technical assistance and consultation in connection with the transfer and implementation of OPKO Know How to TESARO, including the provision and explanation, on request, to TESARO and its Affiliates of all technology, electronic files, materials, reports, data, documents, standard testing requirements, standard operating procedures, notebooks and materials describing or embodying the OPKO Know How. TESARO will be given the reasonable opportunity to meet with, and receive assistance and services of, the OPKOs knowledgeable personnel in connection with TESARO gaining competent knowledge of the contents of the OPKO Know How and OPKOs activities related to Licensed Product. As stated above, OPKO personnel were not involved in the discovery, manufacture, formulation, sourcing, research or development of the Licensed Product or any API and have only gained information relating to the Licensed Product in connection with the Asset Purchase Agreement and its research and development efforts undertaken since the consummation of the Asset Purchase Agreement in November 2009, much of which has been undertaken through the assistance of Third Party consultants. OPKO intended to use Third Parties for, and therefore had not engaged in, the development or formulation of dosage forms or the manufacture of drug product or API in support of the clinical development program or commercialization of Licensed Product. OPKO will seek the assistance of Merck & Co., Inc. to the extent such support continues to be available under Section 2.5 of the Asset Purchase Agreement to provide the Technical Operations Support, including discussion with appropriate Merck & Co., Inc. personnel, as outlined in the Technical Transfer Services included as Schedule 2.5 to the Asset | |||||
| Purchase Agreement, to: | ||||||
| | Identify the identity and location of all archived development samples for transfer to TESARO and/or TESARO designees |
6
| Function | Service to be Provided by OPKO | Comments | ||||
| | Discuss regulatory files and correspondence with regulatory authorities, including an outline of open obligations | |||||
| | Discuss the rationale of Phase 3 dose and formulation and clinical utility of the current IV formulation. Personnel shall also identify the identity and location of all archived study samples for transfer to TESARO and/or TESARO designees. | |||||
| - | Discuss key completed toxicology studies including but not limited to carcinogenicity studies, oral chronic toxicology, development and reproductivity studies, and IV studies, as well as safety pharmacology studies,. Personnel shall also identify the identity and location of all archived study samples for transfer to TESARO and/or TESARO designees. | |||||
| - | Discuss past and current research and development efforts specific for the Licensed Product, including the status of study reports, data and analyses for each study completed as well as available biospecimens for transfer to TESARO and/or TESARO designees. | |||||
| - | Assist in the transfer of the current bioanalytical methods to TESARO and/or any of TESAROS designees, as well as available biospecimens for transfer to TESARO and/or TESARO designees. | |||||
| - | Provide relevant information particularly audit reports related to the Licensed Products to ensure all studies were conducted in accordance with GLP, GMP & GCP, to provide certification that appropriate storage conditions for all inventoried materials has been met at all locations of storage and during all periods of transit, and to provide appropriate documentation to support the chain of custody to OPKO and then to TESARO and/or any of TESAROS designees. | |||||
| - | IT personnel to discuss format, systems utilized and transfer of nonclinical, clinical and CMC data. | |||||
| (Note: all such meetings and communication will be coordinated through the Team Leaders). | ||||||
7
| Function | Service to be Provided by OPKO | Comments | ||||
Regulatory Services
|
OPKO and TESARO shall establish a prompt communication and interaction process to ensure the orderly transfer of all regulatory filings related to Licensed Product (Regulatory Filings) as promptly as practicable following the Effective Date. Within thirty (30) days following the Effective Date, or as otherwise agreed by the Parties, the Parties shall file with the FDA and any other applicable Regulatory Authority, such information as may be required to transfer the Regulatory Filings from OPKO to TESARO. Both OPKO and TESARO agree to use Commercially Reasonable Efforts to take any actions required by the FDA or other applicable Regulatory Authority to affect the transfer of the Regulatory Filings to TESARO. | |||||
Manufacturing Process
|
Seek the assistance of Merck & Co., Inc. under Section 2.5 of the Asset Purchase Agreement towards the development and implementation of a technology transfer protocol for the transfer of the manufacturing process (including in-process methods) and formulation process for API and formulated drug substance for the Licensed Product to TESARO and/or its designees. | |||||
| Seek the assistance of Merck & Co., Inc. under Section 2.5 of the Asset Purchase Agreement to provide training to TESARO and/or TESAROS designees in the manufacturing and testing of the Licensed Product. | ||||||
Intellectual Property
|
OPKO shall transfer to TESARO responsibility for filing and prosecuting any patent applications and patents included in the OPKO Patent Rights and maintaining any patents included in the OPKO Patent Rights, and shall (a) execute any legal documents, such for recordation in any U.S. or foreign offices or agencies, to evidence TESAROs control of prosecution and maintenance of the applicable OPKO Patent Rights, (b) performing all reasonable actions that may be necessary or useful to complete the assignment to TESARO of such responsibilities, and (c) provide reasonable cooperation to TESARO in connection with such activities in accordance with Section 5.1 of the Exclusive License Agreement. | |||||
Part C-Technology Transfer Schedule.
The schedule for performance of certain specified Technology Transfer Services and form of transfer
of certain OPKO Know-how are set forth in the Table I below (the Technology Transfer Schedule).
The
8
Parties representatives comprising the Technical Transfer Team will have the ability, by
mutual written agreement of the Team Leaders from both Parties, to modify in writing the Technology
Transfer Schedule, provided that no such modification shall amend the terms of the Agreement other
than to specifically amend the timelines and format set forth in the Technology Transfer Schedule
unless both Parties agree in writing to amend this Agreement in accordance with Section 11.7 of the
Agreement.
9
Table 1
Technology Transfer Schedule
Technology Transfer Schedule
| Delivery Time (or such later | ||||||
| OPKO Know-how | time as TESARO may request) | Delivery Method | Discussion/Comments | |||
Records related to Licensed
Product in the Data Room
|
OPKO will provide within **** calendar days of the Effective Date | To be delivered in such form as currently exists via commercial carrier. | Will contain the entire contents of the data room that was available to TESARO during due diligence. | |||
IND 77,044
|
OPKO will provide within **** calendar days of the Effective Date | To be delivered on CD or DVD via commercial carrier. | **** which shall, in no event, be greater than 60 calendar days after the Effective Date. | |||
IND 72,754
|
OPKO will provide within **** calendar days of the Effective Date | To be delivered on CD or DVD via commercial carrier. | **** which shall, in no event, be greater than 60 calendar days after the Effective Date. | |||
Regulatory Documents |
||||||
****
|
To be delivered within **** days of the Effective Date. | Hard copy (and electronic as may be available) of all correspondence to and from regulatory agencies and attachments arranged in chronological order. The original IND and IMPD to be delivered on CD or DVD. | ||||
Pharmacovigilance |
||||||
****
|
To be transferred to TESARO within **** calendar days of the Effective Date. | To be delivered on CD or DVD via commercial carrier | **** | |||
Discovery Biology |
||||||
****
|
To be delivered within **** days of the Effective Date. | To be delivered on CD or DVD via commercial carrier | If hard copy signatures were obtained for final report, the original signed report should be provided. Should include any draft reports that may be in process. |
10
| Delivery Time (or such later | ||||||
| OPKO Know-how | time as TESARO may request) | Delivery Method | Discussion/Comments | |||
Drug Metabolism &
Pharmacokinetics |
||||||
****
|
To be delivered within
**** days of the Effective
Date.
|
To be delivered
on CD or DVD via
commercial carrier
|
If hard copy signatures were obtained for final report, the original signed report should be provided. Should include any draft reports that may be in process, as well as associated raw data analyses. | |||
Drug Safety |
||||||
****
|
To be delivered within
**** days of the Effective
Date.
|
To be delivered
on CD or DVD via
commercial carrier
|
If hard copy signatures were obtained for final report, the original signed report should be provided. Should include any draft reports that may be in process, as well as associated raw data analyses. | |||
****
|
To be delivered within
**** days of the Effective
Date.
|
To be delivered
on CD or DVD via
commercial carrier
|
If hard copy signatures were obtained for final report, the original signed report should be provided. Should include any draft reports that may be in process, as well as associated raw data analyses. |
11
| Delivery Time (or such later | ||||||
| OPKO Know-how | time as TESARO may request) | Delivery Method | Discussion/Comments | |||
****
|
To be delivered within **** days of the Effective Date. | To be delivered on CD or DVD as electronic transport file via commercial carrier | Software that may be required to access the data will need to be specified by OPKO. | |||
Early Clinical Research &
Experimental Medicine |
||||||
****
|
To be delivered within
**** days of the Effective
Date.
|
To be delivered
on CD or DVD via
commercial carrier
|
If hard copy signatures were obtained for final report, the original signed report should be provided. Should include any draft reports that may be in process, as well as associated raw data analyses. | |||
****
|
To be delivered within **** days of the Effective Date. | To be delivered on CD or DVD as a SAS transport file via commercial carrier | Software that may be required to access the data will need to be specified by OPKO. |
12
| Delivery Time (or such later | ||||||
| OPKO Know-how | time as TESARO may request) | Delivery Method | Discussion/Comments | |||
Clinical Research |
||||||
****
|
**** - To be delivered within
**** calendar days of the
Effective Date
****
To be delivered within
**** calendar days of the
Effective Date
****
To be delivered within
**** calendar days of the
Effective Date.
|
To be delivered
on CD or DVD via
commercial carrier
Delivery
conditions to be
specified per
sample requirements
|
If hard copy signatures were obtained for final report, the original signed report should be provided. Should include any draft reports that may be in process, as well as associated raw data analyses. | |||
****
|
To be delivered within **** calendar days of the Effective Date. | To be delivered on CD or DVD as a SAS transport file via commercial carrier | Software that may be required to access the data will need to be specified by OPKO. | |||
Chemistry, Manufacturing, and
Controls |
||||||
****
|
To be delivered within **** calendar days of the Effective Date. | To be delivered on CD or DVD via commercial carrier | If hard copy signatures were obtained for final report, the original signed report should be provided. Should include any draft reports that may be in process, as well as associated raw data analyses. |
13
| Delivery Time (or such later | ||||||
| OPKO Know-how | time as TESARO may request) | Delivery Method | Discussion/Comments | |||
****
|
To be delivered within **** calendar days of the Effective Date. | To be delivered on CD or DVD via commercial carrier | ||||
****
|
To be delivered within **** calendar days of the Effective Date. | To be delivered on CD or DVD via commercial carrier | ||||
****
|
To be delivered within **** calendar days of the Effective Date. | To be delivered on CD or DVD via commercial carrier | ||||
****
|
To be delivered within **** calendar days of the Effective Date. | To be delivered on CD or DVD via commercial carrier | **** | |||
|
||||||
Occupational & Environmental Toxicology |
||||||
****
|
To be delivered within **** calendar days of the Effective Date. | To be delivered on CD or DVD via commercial carrier | ||||
Market Research
|
To be delivered within **** calendar days of the Effective Date. | To be delivered on CD or DVD via commercial carrier | ||||
****
|
To be delivered within **** calendar days of the Effective Date. | To be delivered on CD or DVD via commercial carrier |
14
| Delivery Time (or such later | ||||||
| OPKO Know-how | time as TESARO may request) | Delivery Method | Discussion/Comments | |||
****
|
To be delivered within **** calendar days of the Effective Date. | To be delivered on CD or DVD via commercial carrier | ||||
****
|
To be delivered within **** calendar days of the Effective Date. | To be delivered on CD or DVD via commercial carrier | ||||
****
|
To be delivered within **** calendar days of the Effective Date. | To be delivered on CD or DVD via commercial carrier | ||||
****
|
To be delivered within **** calendar days of the Effective Date. | To be delivered on CD or DVD via commercial carrier | ||||
****
|
To be delivered within **** calendar days of the Effective Date. | To be delivered on CD or DVD via commercial carrier | ||||
****
|
To be delivered within **** calendar days of the Effective Date. | To be delivered on CD or DVD via commercial carrier | ||||
Physical chemical inventory |
||||||
API ****
|
To be delivered within **** calendar days of TESARO providing OPKO with the shipping instructions and location, or as otherwise agreed by the parties. | OPKO will ship via commercial carrier under appropriate sample storage and control conditions per sample requirements | **** |
15
| Delivery Time (or such later | ||||||
| OPKO Know-how | time as TESARO may request) | Delivery Method | Discussion/Comments | |||
****
|
To be delivered within **** calendar days of TESARO providing OPKO with the shipping instructions and location, or as otherwise agreed by the parties. | OPKO will ship via commercial carrier under appropriate sample storage and control conditions per sample requirements | ||||
****
|
To be delivered within **** calendar days of TESARO providing OPKO with the shipping instructions and location, or as otherwise agreed by the parties. | OPKO will ship via commercial carrier under appropriate sample storage and control conditions per sample requirements | ||||
****
|
To be delivered within **** calendar days of TESARO providing OPKO with the shipping instructions and location, or as otherwise agreed by the parties. | OPKO will ship via commercial carrier under appropriate sample storage and control conditions per sample requirements | ||||
****
|
To be delivered within **** calendar days of TESARO providing OPKO with the shipping instructions and location, or as otherwise agreed by the parties. | OPKO will ship via commercial carrier under appropriate sample storage and control conditions per sample requirements | ||||
****
|
To be delivered within **** calendar days of TESARO providing OPKO with the shipping instructions and location, or as otherwise agreed by the parties. | OPKO will ship via commercial carrier under appropriate sample storage and control conditions per sample requirements |
16
| Delivery Time (or such later | ||||||
| OPKO Know-how | time as TESARO may request) | Delivery Method | Discussion/Comments | |||
****
|
To be delivered within **** calendar days of TESARO providing OPKO with the shipping instructions and location, or as otherwise agreed by the parties. | OPKO will ship via commercial carrier under appropriate sample storage and control conditions per sample requirements | ||||
Samples |
||||||
****
|
At TESAROs direction, to be provided with **** days of the Effective Date or retained by OPKO | Delivery conditions to be specified per sample requirements | ||||
OPKO Patent Rights |
||||||
All **** (whether ****
prosecution counsel relating
to any or all OPKO Patent
Rights, all **** tile each
issued patent under the OPKO
Patent Rights).
|
Transfer of the patent portfolio will occur within **** calendar days of the Effective Date | To be delivered on CD or DVD or hard copy via commercial carrier | ||||
Notebooks or all other relevant
data or material (even draft
form). |
||||||
Originals or copies of ****
|
To be delivered within **** calendar days of the Effective Date. | To be delivered on CD or DVD or hard copy via commercial carrier | Materials will need to be redacted for information not relevant to the OPKO Know How. |
17
Exhibit E
Form of Press Release
[Attached]

FOR IMMEDIATE RELEASE
TESARO and OPKO Health Sign Exclusive License Agreement for Rolapitant
| | Rolapitant is a Phase III-ready neurokinin-l (NK-l) receptor antagonist in development for chemotherapy induced nausea and vomiting (CINV) | ||
| | TESARO responsible for worldwide development and commercialization of rolapitant |
Boston, MA and Miami, FL -December 13, 2010 -TESARO, Inc. and OPKO Health, Inc. (NYSE Amex:OPK),
today announced that they have signed a definitive agreement granting TESARO exclusive rights to
the development, manufacture, commercialization and distribution of rolapitant and a related
compound. Rolapitant is a potent and selective neurokinin-1 (NK-1) receptor antagonist with an
extended plasma half-life that has the potential to improve the management of nausea and vomiting
experienced by cancer patients undergoing chemotherapy. Rolapitant, which is Phase III ready,
demonstrated promising efficacy in Phase II testing for prevention of nausea and vomiting in
patients undergoing highly emetogenic chemotherapy.
Under the terms of the agreement, OPKO will acquire an approximately 10% equity investment in
TESARO. OPKO is eligible for payments of up to over $120 million, including an up-front payment and
additional payments based upon achievement of specified regulatory and commercialization
milestones; in addition, OPKO will receive tiered royalties on sales. Under the agreement, OPKO and
TESARO will share future profits from the commercialization of licensed products in Japan and OPKO
will have an option to market the products in Latin America.
TESARO is very pleased to announce this agreement with OPKO and to advance the development of this
important product candidate, rolapitant, said Lonnie Moulder, Chief Executive Officer of TESARO.
Our leadership team has a deep understanding of the unmet need that still exists in oncology
supportive care, given our successful commercialization of the market-leading therapy for CINV
prevention at the helm of MGI PHARMA. We believe that rolapitant is a differentiated product with
great potential to help cancer patients undergoing chemotherapy.
TESARO was co-founded by former executives of MGI PHARMA, an oncology and acute-care focused
specialty biopharmaceutical company that Eisai Co., Ltd. acquired in 2008 for $3.9 billion. While
at MGI PHARMA, TESARO executives led the clinical development and commercialization of numerous
drugs, including commercialization of Aloxi® (palonosetron HCI), the leading drug in the 5-HT3
receptor antagonist class for prevention of CINV.
We are pleased to complete this important transaction and look forward to seeing rolapitant
progress towards registration in key markets throughout the world, said Phillip Frost, M.D.,
OPKOs Chairman and Chief Executive Officer. The TESARO teams successful experience with the
development and commercialization of oncology supportive care products will be of special benefit
in making rolapitant a commercial success.
About Rolapitant:
Rolapitant, a potent and selective neurokinin-1 (NK-1) receptor antagonist with an extended plasma
half-life, has completed Phase II clinical testing for prevention of chemotherapy induced nausea
and vomiting indications. NK-1 receptors are highly concentrated in the brain and bind substance P,
a neurokinin that elicits an emetogenic response. Activation ofNK-1 receptors plays a central role
in nausea and vomiting induced by emetogenic cancer chemotherapy.
About Chemotherapy Induced Nausea and Vomiting (CINV):
CINV is estimated to afflict over 70% of cancer patients undergoing chemotherapy and, if not
prevented, may possibly result in a delay or even discontinuation of chemotherapy treatment. NK-1
receptor antagonists have been demonstrated to improve the management of nausea and vomiting
experienced by cancer patients undergoing chemotherapy.
About OPKO Health, Inc.
Miami-based OPKO is a specialty healthcare company involved in the discovery, development, and
commercialization of proprietary pharmaceutical products, medical devices, vaccines, diagnostic
technologies and imaging systems. Initially focused on the treatment and management of ophthalmic
diseases, OPKO has since expanded into other areas of major unmet medical need. For more
information, visit www.opko.com.
About TESARO, INC.
Founded in 2010, TESARO is a privately held oncology-focused biopharmaceutical company dedicated to
improving the lives of cancer patients by developing and commercializing safer and more effective
therapeutics. Earlier this year, TESARO secured $60 million in start-up funding from New Enterprise
Associates (NEA) and the TESARO founders. TESARO is headquartered in Boston, Massachusetts. For
more information, visit www.tesarobio.com.
For Further Information Contact:
| For TESARO | For OPKO Health | |
Richard Rodgers
|
Steve Rubin | |
EVP & Chief Financial Officer
|
EVP -Administration | |
+1.339.970.0903
|
+1.305.575.6015 | |
rrodgers@tesarobio.com
|
srubin@opko.com |
This press release contains forward-looking statements, as that term is defined under the
Private Securities Litigation Reform Act of1995 (PSLRA), which statements may be identified by
words such as expects, plans, projects, will, may, anticipates, believes,
should, intends, estimates, and other words of similar meaning, including statements
regarding product development efforts, including the ability to develop and commercialize rolapitant for
chemotherapy-induced nausea and vomiting, the ability to obtain registration for rolapitant in
key markets and the timing thereof, and the potential for rolapitant to help cancer patients
undergoing chemotherapy, as well as other non-historical statements about expectations, beliefs
or intentions regarding business, technologies and products, financial condition, strategies or
prospects. These forward-looking statements are not guarantees of OPKOs or TESAROs future
performance and involve a number of risks and uncertainties that may cause actual results to
differ materially from the results discussed in these statements. Many factors could cause either
Companys actual activities or results to differ materially from the activities and results
anticipated in forward-looking statements. These factors include, an inability to successfully
develop and commercialize rolapitant and the NK-1 program assets, that rolapitant may not
achieve the expected results or effectiveness and may not generate data that would support the
approval or marketing of this product, that others may develop products, including other NK-1
receptor antagonists, which are superior to rolapitant, and that the acquired compounds may not
have advantages over presently marketed products. In addition, forward-looking statements may
also be adversely affected by risks inherent in funding, developing and obtaining regulatory
approvals of new, commercially-viable and competitive products and treatments, general market
factors, competitive product development, product availability, federal and state regulations and
legislation, the regulatory process for new products and indications, manufacturing issues that
may arise, patent positions and litigation, among other factors. The forward-looking statements
contained in this press release speak only as of the date the statements were made, and OPKO
and TESARO do not undertake any obligation to update forward-looking statements. The
Companies intend that all forward-looking statements be subject to the safe-harbor provisions of
the PSLRA.
###
Exhibit F
Material Agreements
Asset Purchase Agreement, dated October 12, 2009, by and among Schering Corporation and OPKO Health,
Inc., as amended by letter agreement dated June 29, 2010
Clinical Research Services Agreement, dated October 7, 2010, by and among OPKO Health, Inc. and Pharm-Olam International, Ltd.
Clinical Research Services Agreement, dated October 7, 2010, by and among OPKO Health, Inc. and Pharm-Olam International, Ltd.
Clinical Research Services Agreement, dated October 7, 2010, by and among OPKO Health, Inc. and Pharm-Olam International, Ltd.
Archiving Agreement, dated April 29, 2010, by and among OPKO Health, Inc. and EPL Pathology Archives, Inc.
Cost Proposal Regarding Retention of Radiolabeled and Stable Isotope Labeled Test Articles, dated
June 7, 2010, by and among OPKO Health, Inc. and XenoBiotic Laboratories, Inc.
Consulting Agreement, dated October 9, 2009, as amended on October 15, 2010, by and among OPKO
Health, Inc. and SNC Partners LLC.
Consulting Agreement, dated October 9, 2009, as amended on October 15, 2010, by and among OPKO
Health, Inc. and Scott Reines, M.D., Ph.D.
Consulting Agreement, dated November 30, 2009, by and among OPKO Health, Inc. and Keith A. Candiotti, M.D.
Consulting Agreement, dated February 11, 2010, by and among OPKO Health, Inc. and Goldmann Consulting LLC.
Consulting Agreement, dated September 15, 2010, by and among OPKO Health, Inc. and Steven M. Grunberg, M.D.
Consulting Agreement, dated October 11, 2010, by and among OPKO Health, Inc. and INDAPharma, LLC.